Several studies have examined whether olanzapine has superior efficacy. In a retrospective analysis, olanzapine was shown to be more effective than haloperidol for the treatment of positive and negative symptoms in patients who met retrospective and cross-sectional criteria for treatment resistance
(4). Tollefson and colleagues
(5) conducted a noninferiority study to demonstrate the clinical equivalence of olanzapine and clozapine. They found no significant difference in efficacy between the two drugs.
In contrast, Conley and colleagues
(6) conducted a study in which they used similar entry criteria and study design as the Kane et al. multicenter study that demonstrated superior efficacy of clozapine in treatment-resistant patients
(1). Conley et al.
(7) found no difference between olanzapine and chlorpromazine in treatment-resistant inpatients. Patients who failed to respond to olanzapine were subsequently treated with open-label clozapine. Forty-one percent of the patients treated with clozapine responded, with significant improvement in Brief Psychiatric Rating Scale (BPRS)
(8) measures of positive and negative symptoms.
Finally, Volavka and colleagues
(9) compared olanzapine, risperidone, clozapine, and haloperidol in inpatients with treatment-resistant schizophrenia and found no significant differences among the four drugs for positive symptoms. Olanzapine was superior to haloperidol for negative symptoms, but the difference between the two treatment groups was attributable, in part, to worsening of negative symptoms in the haloperidol-treated patients.
The current study was designed to examine prospectively the comparative efficacy of olanzapine and haloperidol for positive and negative symptoms in partially responsive outpatients with schizophrenia.
Method
Subjects
Patients meeting DSM-IV criteria for schizophrenia or schizoaffective disorder were selected from the Maryland Psychiatric Research Center Outpatient Research Program and the VISN 5 Mental Illness Research, Education, and Clinical Center Psychopharmacology Clinic for entry into the study. Patients were diagnosed by using a best-estimate approach that used all available information from a structured diagnostic interview (the Structured Clinical Interview for DSM-IV
[10]), direct assessment, family informants, and past medical records. Patients with concurrent drug abuse or alcoholism, organic brain disorders, or mental retardation were excluded from the study. All patients provided written informed consent before participating in the study.
Patients were required to meet retrospective and prospective criteria for partial response to conventional antipsychotics. Retrospective criteria were 1) a history of residual positive and/or negative symptoms after at least two 6-week trials of therapeutic doses of conventional antipsychotics from at least two different classes; and 2) a minimum level of positive and/or negative symptoms at the time of evaluation for participation in the study.
The minimum positive symptom level was a total score of 8 or more on the four BPRS positive symptom items
(8) or a score of 4 or more on any one of the items. The four BPRS positive symptom items are conceptual disorganization, hallucinations, unusual thought content, and suspiciousness. BPRS item scores range from 1 to 7. The minimum negative symptom level was a total score of 20 or more on the Scale for the Assessment of Negative Symptoms (SANS)
(11) or a score of 2 or more on at least one of four SANS global items (i.e., affective flattening, alogia, avolition/apathy, or anhedonia/asociality). The SANS total score included all items except inappropriate affect, including poverty of content of speech, social inattentiveness, inattentiveness during mental status testing, and all global items. SANS item scores range from 0 to 5.
The prospective evaluation of partial responsiveness consisted of a 4-week trial of 20 mg/day of open-labeled fluphenazine, with dose adjustments allowed between 10 and 30 mg/day. Subjects were excluded from the double-blind study if they demonstrated a 30% or greater improvement in positive or negative symptoms, no longer met the minimal level of positive or negative symptom criteria, relapsed, or were intolerant of fluphenazine.
Clinical Assessments
Patients were categorized into subgroups with and without the deficit syndrome by using the Schedule for the Deficit Syndrome
(12), a semistructured interview that provides specific criteria for assessing the presence of negative symptoms, the duration of symptoms, and whether the symptoms are primary or secondary. Additional information is obtained from clinicians with longstanding contact with the patients and from family members. The interrater agreement kappa for global categorization was 0.73
(12). The four BPRS positive symptom items and the modified SANS total score were used to assess positive and negative symptom change, respectively. The Clinical Global Impression (CGI) severity of illness item was used to assess global changes. The BPRS, SANS, and CGI ratings were obtained weekly. The Hamilton Depression Rating Scale
(13) was obtained biweekly to assess change in depressive symptoms. Social and occupational functioning and quality of life were assessed by using the Level of Functioning Scale
(14) and Quality of Life Scale
(15), respectively; these ratings were obtained at baseline and at the end of the double-blind study. The symptom and functioning ratings were conducted by master’s and doctoral level clinicians. Intraclass correlation coefficients (ICCs) for these instruments ranged from 0.76 to 0.90.
The Simpson-Angus Rating Scale
(16) and the Maryland Psychiatric Research Center Tardive Dyskinesia Scale
(17) were used to assess extrapyramidal symptoms and dyskinetic movements, respectively. The ICC was 0.90 for the Simpson-Angus Rating Scale and 0.89 for the Maryland Psychiatric Research Center Tardive Dyskinesia Scale. The Simpson-Angus Rating Scale was administered weekly and the Maryland Psychiatric Research Center Tardive Dyskinesia Scale every 4 weeks during the double-blind study by research nurses. The Side Effect Checklist was used to assess side effects and monitor vital signs. The Side Effect Checklist comprises 22 common side effects, which are rated on a scale of 1 (none) to 4 (severe). These ratings were made by a nonblind pharmacist. All raters other than the nonblind pharmacist were blind to treatment assignment and deficit/nondeficit categorization.
Study Design
Patients who met the retrospective criteria for partial response and continued to meet admission criteria on completion of the 4-week open-label fluphenazine trial were randomly assigned to a 16-week double-blind, parallel-groups comparison of olanzapine versus haloperidol. Olanzapine and haloperidol were each initiated at 15 mg/day; fluphenazine was gradually tapered off over the first 2 weeks of the study. Olanzapine and haloperidol doses could be adjusted within fixed limits (olanzapine: 10–30 mg/day; haloperidol: 10–30 mg/day), either to maximize efficacy or to minimize side effects. Benztropine (4 mg/day) was prescribed for patients randomly assigned to receive haloperidol to minimize extrapyramidal symptoms and the potential for revealing treatment assignment. The benztropine dose could be adjusted between 0 and 6 mg/day. Patients randomly assigned to receive olanzapine were given placebo benztropine.
Patients were seen biweekly for clinical ratings and safety evaluations. A patient was withdrawn from the study if he or she met the following criteria for clinically significant symptom exacerbation: 1) the patient was judged to be entering an exacerbation of his or her illness by the treating clinician and 2) one or more of the following: a) relative to the most recent BPRS ratings, an increase of 3 points or more or an increase from a score of 6 to a score of 7 on any of the following BPRS items: somatic concern, conceptual disorganization, hostility, suspiciousness, or hallucinatory behavior, or b) an increase of 2 or more on the CGI global item or an increase in score from 6 to 7.
Medication compliance was assessed weekly by a pill count and medication review. In addition, all patients had a compliance plan that consisted of medication checks by family and/or mental health care providers who had extensive contact with the patients. All patients who were judged to have received 75% or more of their assigned study medication were considered compliant.
Statistical Analyses
Treatment differences in repeated assessments of psychiatric symptoms (BPRS total and positive symptom scores, SANS total score, Hamilton depression scale total score, and CGI) were tested by using the generalized estimating equations method for unbalanced repeated-measures analysis of covariance (ANCOVA)
(18), in which mean follow-up score is estimated from the main effects of treatment assignment, time, and deficit/nondeficit categorization, adjusted for the baseline score. A preliminary test for the presence of a treatment-by-deficit/nondeficit categorization interaction was conducted, and main effects from a model without the interaction were reported if the interaction was not statistically significant. Treatment differences in weight, pulse, and diastolic and systolic blood pressure were analyzed similarly by using generalized estimating equations method ANCOVA, with adjustments for baseline value and deficit/nondeficit categorization. For descriptive purposes, we present mean values by treatment for these symptom and physiological measures at baseline and last study visit, along with the test results from the generalized estimating equations method models.
Treatment differences in Simpson-Angus Rating Scale total score and Maryland Psychiatric Research Center Tardive Dyskinesia Scale total score were tested as follows: for each patient, the baseline rating and all available double-blind follow-up ratings were used to calculate Kendall’s tau-b correlation between visit and total score. A positive tau-b correlation for a given patient is interpreted as evidence that the Simpson-Angus Rating Scale (or Maryland Psychiatric Research Center Tardive Dyskinesia Scale) total score tended to increase from baseline over the course of follow-up. Wilcoxon tests were then used to compare the distribution of tau-b scores in the two treatment groups. The same procedure was used to compare trends in repeated ratings of presence and severity of adverse effects listed on the Side Effect Checklist. We adopted this technique following the observations of Arndt and colleagues
(19) that 1) the distribution of extrapyramidal symptoms ratings typically shows large numbers of patients clustered persistently at low values, with a relatively small proportion of patients showing both high scores and high rating-to-rating variability; 2) this distribution fails to meet several important statistical assumptions of repeated-measures analysis of variance and related parametric techniques, suggesting the need for alternate nonparametric analyses. In a later paper examining Simpson-Angus Rating Scale and similar psychiatric ratings, Arndt and colleagues
(20) reported that using the Kendall tau-b correlation to summarize participants’ clinical course provides superior power to commonly used alternatives summary indexes for detecting differences among patient groups. For descriptive purposes, we present means by treatment on the Simpson-Angus Rating Scale and Maryland Psychiatric Research Center Tardive Dyskinesia Scale total scores at baseline and last study visit, along with the Wilcoxon test results comparing the Kendall tau-b correlations with visit.
Results
Sixty-eight patients entered the 4-week open-label fluphenazine trial. Sixty-three patients completed the 4-week trial, and none met improvement criteria. Of the five patients who failed to complete this phase, four patients had a clinically significant worsening of their symptoms and one patient chose to leave the clinic. Sixty-three patients entered the double-blind phase. Thirty-four patients were randomly assigned to receive haloperidol and 29 to receive olanzapine. There were three noncompleters in each treatment group. In the olanzapine group, two patients met criteria for a clinically significant worsening of their symptoms and one patient was withdrawn because of alcohol abuse. In the haloperidol group, three patients met criteria for a clinically significant worsening of their symptoms.
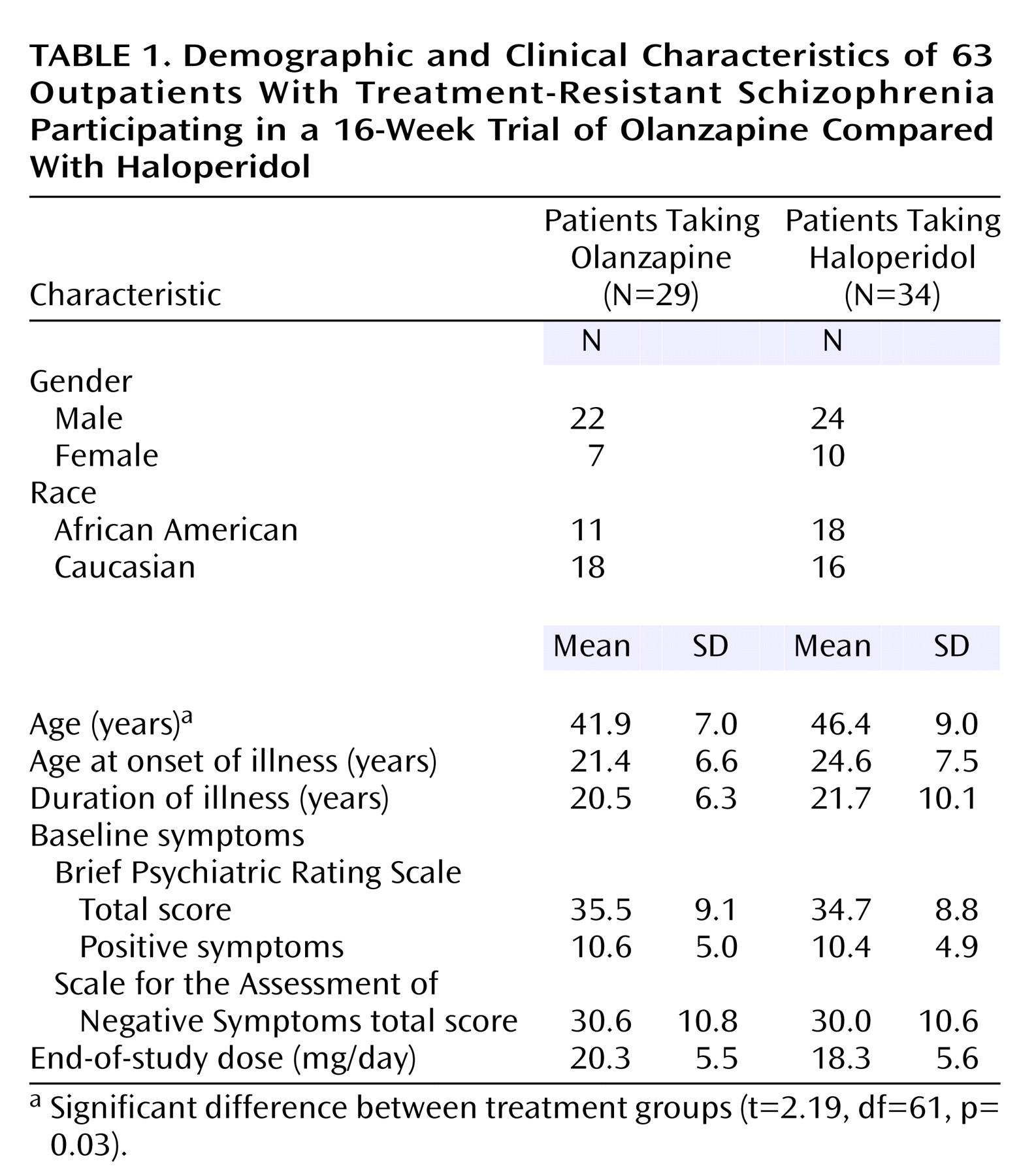
The demographic and baseline clinical characteristics of the patients who entered the double-blind phase are presented in
Table 1. There was a significant age difference between the two groups. There were no other significant differences in demographic, clinical, or baseline symptom characteristics.
Efficacy Measures
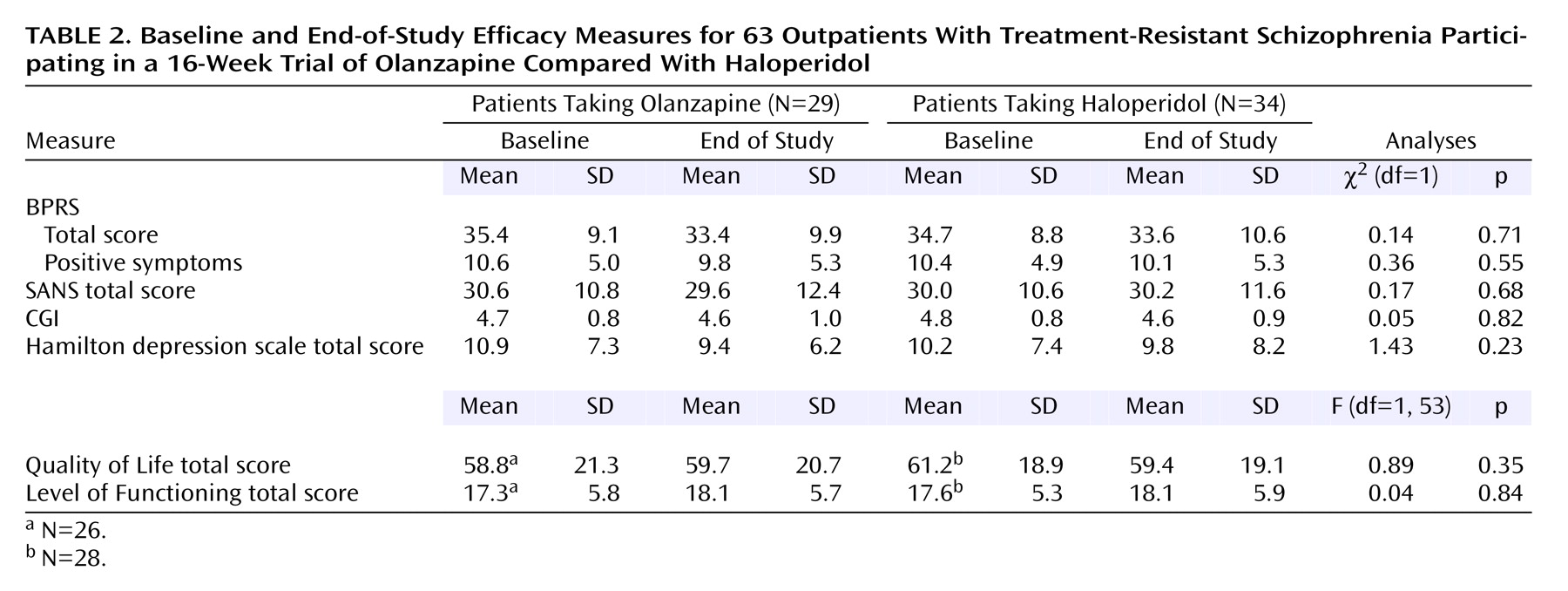
There were no significant differences between patients receiving olanzapine and those receiving haloperidol on the three primary efficacy measures: BPRS total score, score on the BPRS positive symptom items, or SANS total score (
Table 2).
There was a significant interaction between deficit/nondeficit categorization and treatment for BPRS positive symptom items (χ2=4.02, df=1, p<0.05). However, follow-up tests of estimated treatment group differences in positive symptom response were not significant among either the patients with (mean=0.9, SD=0.7) (χ2=1.74, df=1, p=0.19) or without (mean=–1.0, SD=0.6) (χ2=2.59, df=1, p=0.11) the deficit syndrome. There were no significant interactions between deficit/nondeficit categorization and treatment assignment for BPRS total score or SANS total score.
There were no significant group differences or significant interactions between deficit/nondeficit categorization and treatment assignment for the CGI severity item or total Hamilton depression scale score. There were no significant group differences or significant interactions between deficit/nondeficit categorization and treatment assignment for the two measures of social and occupational functioning: the Quality of Life Scale total score and the Level of Functioning Scale total score (
Table 2).
Safety Measures
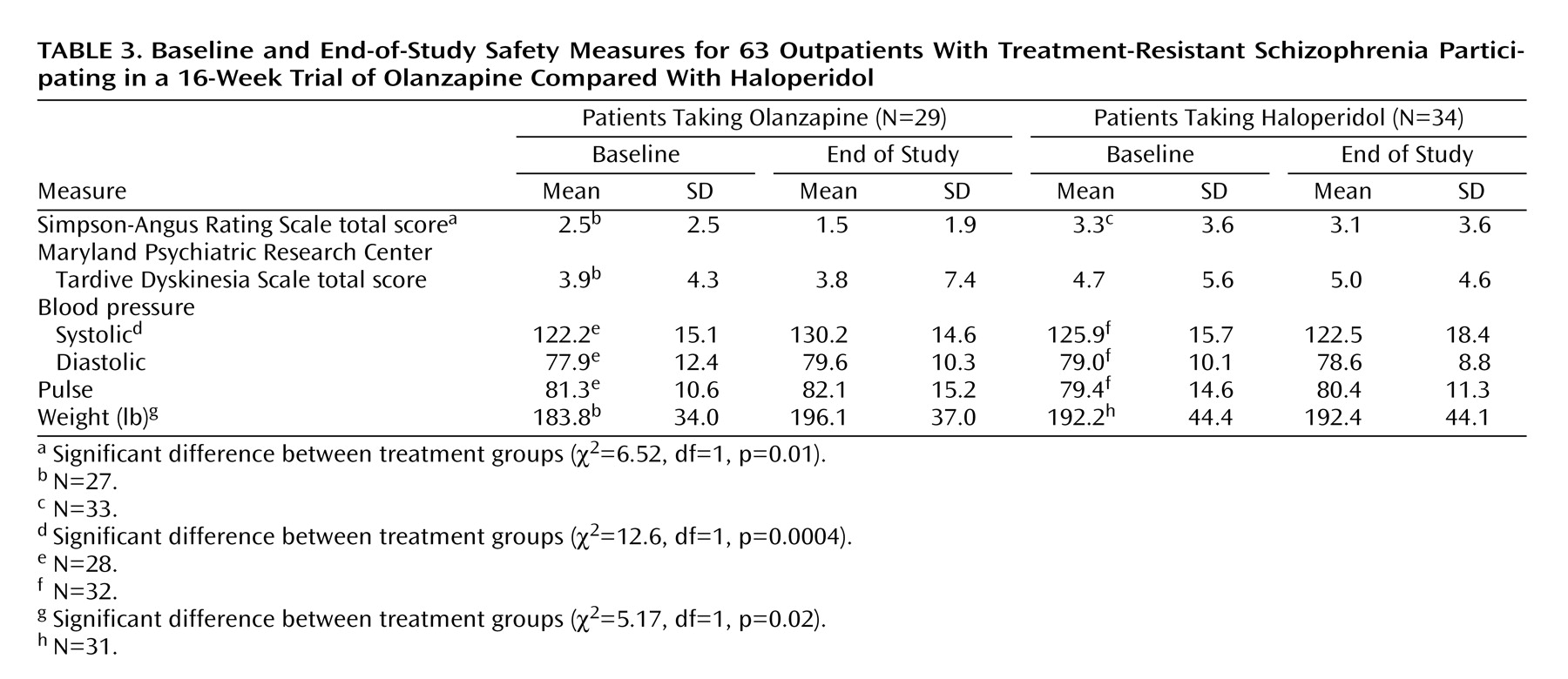
Patients treated with olanzapine had a significantly greater reduction of extrapyramidal symptoms than patients treated with haloperidol (
Table 3). There was no significant group difference in change in dyskinetic movements.
The increases in systolic blood pressure and weight in olanzapine-treated patients were significantly greater than they were in haloperidol-treated patients. There was a significant correlation between change in weight and change in systolic blood pressure in olanzapine-treated patients (r=0.48, p=0.01). This relationship was not significant in haloperidol-treated patients (r=0.23, p=0.21). There were no significant group differences in diastolic blood pressure or pulse.
On the Side Effect Checklist, there were significant group differences on two side effects: stiffness (olanzapine: baseline mean=1.39, SD=0.57, versus end-of-study mean=1.07, SD=0.38; haloperidol: baseline mean=1.19, SD=0.40, versus end-of-study mean=1.28, SD=0.58) (χ2=7.69, df=1, p=0.006) and dry mouth (olanzapine: baseline mean=1.78, SD=0.99, versus end-of-study mean=1.21, SD=0.50; haloperidol: baseline mean=1.65, SD=0.83, versus end-of-study mean=1.78, SD=0.79) (χ2=9.50, df=1, p=0.002). Olanzapine-treated patients had significantly greater reductions in both side effects than haloperidol-treated patients.
Discussion
The study results suggest that olanzapine does not exhibit superior efficacy for positive or negative symptoms in outpatients with partially responsive schizophrenia. There were no significant group differences for either of these symptoms. The lack of evidence of superior efficacy for positive or negative symptoms is in contradistinction to some
(4,
5), but not all
(6,
9), previous studies in these patients. The conflicting results may reflect differences in the procedures used to define the subjects. In the current study, we used essentially the same study design as in our clozapine study
(3), in which we were able to document superior efficacy for positive symptoms, no differential effect for negative symptoms, and long-term efficacy for social and occupational functioning—results that have been confirmed in multiple other studies
(1,
2,
21,
22).
In contrast, in the Breier and Hamilton study
(4), patients were retrospectively selected from a larger parent study designed to assess the acute efficacy of olanzapine
(23). Patients were included in the parent study if they were experiencing substantial symptoms while not taking any antipsychotic medication or while on their current antipsychotic regimen, regardless of adequacy of the trial; if they had experienced a recent side effect associated with their current antipsychotic medication; and if they met cross-sectional symptom severity criteria. Patients were excluded from the parent study if they had failed to respond to three adequate trials of antipsychotics within the last 2 years. There was no prospective assessment of treatment resistance. These latter two design features, in particular, raise questions about how representative the study subjects were of the population of treatment-resistant patients. Indeed, the haloperidol-treated patients, who were included in the putative treatment-resistant group, had a numerically greater positive symptom response than the total study group, which is in contradistinction to previous studies of conventional antipsychotics in treatment-resistant patients
(1,
3,
6).
In the Tollefson et al. study
(5), the selection criteria also allowed for the inclusion of treatment-intolerant patients and there was no prospective evaluation of treatment resistance. In addition, the study design failed to include a conventional antipsychotic comparison arm, which is required to confirm the treatment-resistant categorization. The failure to define adequately a treatment-resistant group will undermine the ability to detect a superior effect of clozapine, since the superior efficacy of clozapine is most apparent in study groups that meet criteria for treatment resistance
(24).
Our results are consistent with the two other studies that used a comparable study design
(6,
9). In the current study, the olanzapine-treated patients had an 8% reduction in positive symptoms and a 3% reduction in negative symptoms. In comparison, Conley and colleagues
(6) reported a 9% reduction in positive symptoms and a 4% reduction in negative symptoms, and Volavka and colleagues
(9) reported a 13% reduction in positive symptoms and a 7% reduction in negative symptoms. The magnitude of the positive symptom response with olanzapine is relatively consistent across the three studies. The positive symptom response in the conventional antipsychotic comparator arms in these studies ranged between 2% and 6%. There is the possibility that a study with a larger number of subjects might demonstrate a significant difference between olanzapine and a conventional antipsychotic for positive symptoms in treatment-resistant patients. However, the magnitude of the positive symptom response in these studies is relatively modest and unlikely to be clinically significant in larger groups. Furthermore, it is considerably less than that observed in the Kane et al. multicenter study
(1) (26% positive symptom reduction) and in our previous outpatient clozapine study
(3) (19% positive symptom reduction). The negative symptom response was small across all three studies.
There were no significant group differences on the global measures of response (i.e., BPRS total score and CGI) or depressive symptoms. However, the study was not designed to examine the comparative efficacy of olanzapine for depressive symptoms, and differential efficacy for these symptoms may have become apparent if patients had been selected for severity of depressive symptoms.
Finally, there were no significant differences between the two drugs on measures of functional outcome. The possibility exists that a longer trial duration would have provided more opportunity to observe an effect on these measures. In our 1-year longitudinal study of clozapine
(3), there was progressive improvement over the follow-up period. However, there was little evidence of any effect of olanzapine or haloperidol on the Level of Functioning Scale or Quality of Life Scale scores.
The two groups did differ on a number of safety measures. Olanzapine-treated patients exhibited a significantly greater reduction than haloperidol-treated patients in extrapyramidal symptoms. The differential effect on extrapyramidal symptoms was also reflected in the significant group difference in subjective complaints of stiffness. The benefit of olanzapine for extrapyramidal symptoms has been previously reported
(23) and represents an important advantage in tolerability of the drug. The two groups also differed in complaints of dry mouth, which probably reflects the use of benztropine in the haloperidol-treated patients. In contrast, olanzapine-treated patients had a mean weight gain of more than 12 pounds during the course of the 16-week study. They also had a significant increase in systolic, but not diastolic, blood pressure. The problem of differential weight gain with olanzapine has been previously reported
(25). Weight gain has been implicated as a major risk factor for hyperlipidemias and the development of hypertension and type II diabetes mellitus
(26,
27). In the current study, weight was significantly correlated with change in systolic blood pressure. The magnitude of weight gain and possible consequences may potentially offset the more benign extrapyramidal symptom profile of olanzapine.
There are several potential limitations of the current study. First, the number of subjects included in the study was relatively small, which could lead to an unreliable estimate of the real effect size. However, the concordance across different studies in the magnitude of positive and negative symptom reduction suggests that the effect size estimate is valid. Second, the olanzapine dose used in the current study may be below the optimally effective dose in this group of patients. The mean dose is well within the recommended range, but, in general, treatment-resistant patients do not respond well to the lower end of the effective dose range. In the study of Volavka et al.
(9), the mean end-of-study olanzapine dose was 30.4 mg/day (SD=6.6), and olanzapine-treated patients experienced a slightly greater symptom response. Third, although the patients met criteria for residual symptoms, they had a relatively low level of positive symptom severity. The relatively low level of positive symptoms could potentially limit the ability to detect change. The use of the same design in our clozapine study, in which we were able to detect a significant reduction in positive symptoms, and the comparability of our results with other studies argue against this possibility.
In summary, in combination with previous studies
(6,
7,
9), these results suggest that olanzapine has limited superior efficacy for either positive or negative symptoms in patients with treatment-resistant schizophrenia.