Mild cognitive impairment may be defined as a transitional state between normal aging and Alzheimer’s disease in which memory impairment is greater than expected for age, but general cognitive function and daily living activities are preserved
(1). The rate of progression to dementia is considerable according to estimates from various longitudinal studies: 12% per year for 4 years
(1), 40% over 2 years
(2), 53% over 3 years
(3), 34%–100% over 4–5 years
(4,
5), and 100% over 9.5 years
(6).
Currently, there has been open discussion about terminology and on whether mild cognitive impairment must be considered either a separate nosological entity at increased risk of dementia or a prodrome of Alzheimer’s disease
(7). Whatever the option, it is of paramount importance to develop tools that enable us to predict conversion to dementia.
The availability of drug therapy for mild-to-moderate Alzheimer’s disease has led to an emphasis on the early detection and management of this disorder. As therapies with the potential to halt progression of Alzheimer’s disease are developed, identification of people with pre-Alzheimer conditions has become increasingly important to determine whether such individuals can benefit from interventional agents.
Previous reports have pointed to the major role of the hippocampal complex in memory
(8,
9). Temporal atrophy and especially hippocampal atrophy have been detected in early Alzheimer’s disease
(10,
11). Another report concluded that memory dysfunction was highly correlated with atrophy of the amygdala-hippocampal complex and of the temporal lobes
(12). Magnetic resonance imaging-based hippocampal volume measurements in patients with mild cognitive impairment have demonstrated that anterior hippocampal formation is most affected in comparison with normal aging, and that the left parahippocampal gyrus shrinks disproportionately to the right
(13). Longitudinal assessments of hippocampal atrophy rate support the hypothesis of a continuum from normal aging to mild cognitive impairment and Alzheimer’s disease
(14). Hippocampal atrophy has been regarded as a significant predictor of dementia in subjects with mild cognitive impairment
(15).
Proton magnetic resonance spectroscopy (
1H-MRS) studies have found decreased
N-acetylaspartate and increased
myo-inositol in the occipital, temporal, parietal, and frontal regions of patients with Alzheimer’s disease even at the early stages of Alzheimer’s disease
(16).
N-Acetylaspartate is one of the most abundant amino acids in the central nervous system, located predominantly in neurons, axons, and dendrites. Its function is not well known so far and remains speculative. It has been thought to play a role in lipid and protein synthesis, but it also may be a product of
N-acetylaspartyl-glutamate degradation or an osmolyte. Because this metabolite is decreased in some neurological diseases such as degenerative dementia, it has been labeled as a surrogate marker of neuronal integrity
(17). The sugar-alcohol compound
myo-inositol may act as an osmoregulator, intracellular messenger, and detoxication agent; it is also regarded as marker of glial cells
(18).
Relative to healthy subjects, a reduced concentration of
N-acetylaspartate has been found in the medial temporal lobe (21% difference) and the cortex of the parietal lobe (13% difference) of patients with Alzheimer’s disease as well as a smaller hippocampus (29% difference) without significant differences between both sides
(19). Poor performance on recognition memory tests was correlated with low gray matter N-acetyl compounds and increased choline concentrations in Alzheimer’s disease patients
(20). Significantly lower
N-acetylaspartate/creatine ratios were found in both hippocampi of 34 Alzheimer’s disease patients in comparison with 22 healthy subjects
(21), but in another study comparing Alzheimer’s disease patients and healthy subjects the differences were only significant for the left hippocampus
(22). However, to our knowledge there have been no longitudinal studies that used
1H-MRS in patients with mild cognitive impairment as a predictor of conversion to Alzheimer’s disease.
On the basis of these reports, we hypothesized that hippocampal alterations in patients with mild cognitive impairment detected with 1H-MRS would predict conversion to Alzheimer’s disease when the patients are followed longitudinally. It is also likely that these alterations may appear early in the occipital and parietal cortices.
Method
The patients we see in our outpatient clinics are usually referred from the community by family physicians. Given the high proportion of elderly people in our area, mild cognitive impairment and dementia of Alzheimer type are relatively frequent diagnoses in our outpatient clinics.
Before the beginning of the study we asked the general practitioners to refer to us all elderly patients with recent memory complaints. From this group we recruited a cohort of 59 consecutive incident patients who fulfilled the criteria of amnestic mild cognitive impairment proposed by Petersen et al.
(1): memory complaints, normal activities of daily living, normal general cognitive function, and abnormal memory for age but no dementia. We also gave special importance to the fact that the memory inefficiencies of the patient had to be corroborated by an informant (wife/husband or relative).
In the clinical history we asked for family history of dementia, vascular risk factors, depression, and other diseases. The final diagnosis was made by taking into account as much information as possible from all sources (general interview, neuropsychological examination, informant report). We only considered patients with a disease duration of up to 12 months as being incident cases of mild cognitive impairment.
Neuropsychological examination at baseline included the Spanish version of the Mini-Mental State Examination (MMSE) (which has a maximum score of 35 points and a cutoff point of 23 for elderly subjects
[23]), Blessed Dementia Rating Scale, and category fluency test (Set Test). Memory was explored with subscales of the 144 Battery of Signoret (Spanish validated version)
(24):
1.
Immediate verbal recall of a maximum of 12 words with three trials, as well as delayed recall of those words. The maximum possible score is 12 points for immediate recall and 12 for delayed recall.
2.
Immediate visual recall: the patient has to reproduce a complex geometric figure after 1 minute of observation. Delayed visual recall was also evaluated. The maximum possible score is 24 points for each.
3.
Logical memory: immediate and delayed recall of a brief story. Maximum score=24 points for each.
4.
Digit span (number of digits forward).
Memory functions were also assessed in a random comparison group consisting of 20 healthy elderly subjects recruited from the same population so as to demonstrate baseline differences between patients with mild cognitive impairment and subjects without mild cognitive impairment and to establish the cutoff point. We defined abnormal memory as a total memory score <60, which represented a difference greater than one standard deviation from the mean of healthy subjects.
The patients were also evaluated with the Clinical Dementia Rating Scale and the Global Deterioration Scale for staging purposes. Depression was initially assessed by means of the Geriatric Depression Scale
(25). Laboratory tests included standard blood tests; measurement of sedimentation rate, cell count, and levels of vitamin B
12, folic acid, and TSH; a proteinogram; and serologic tests for syphilis. The final diagnosis of amnestic mild cognitive impairment was made when the patients fulfilled the criteria of Petersen et al.
(1) and an informant’s report corroborated the memory complaints. We interpreted with caution some low scores yielded in neuropsychological examination, since some patients had a low level of education. Scores higher than 60 on the memory scale did not exclude the diagnosis of mild cognitive impairment if the clinical history and caregiver report were clearly compatible with memory loss.
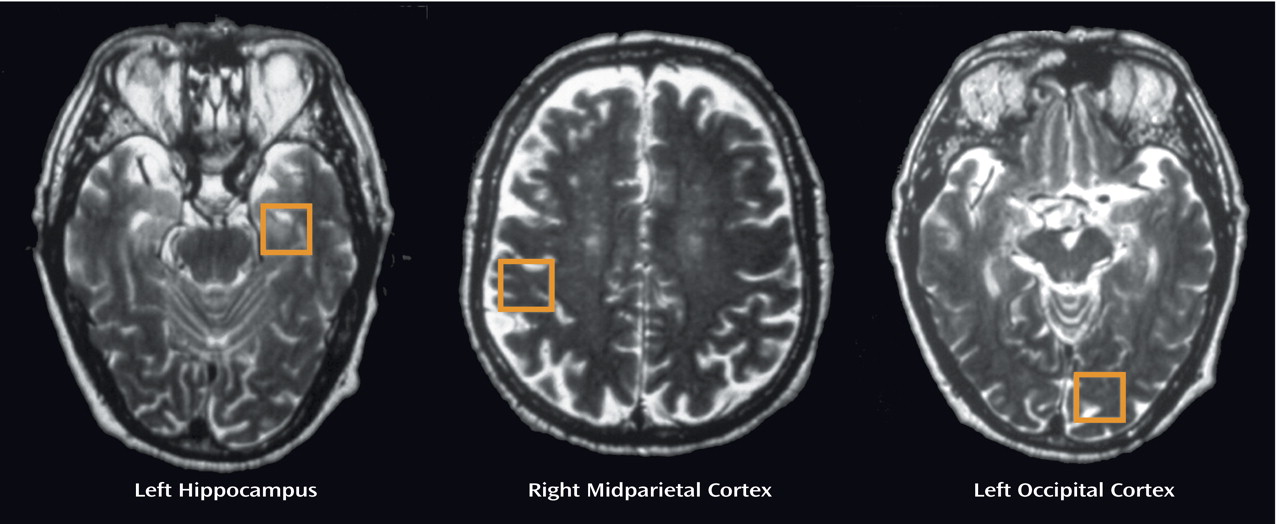
Upon completion of the clinical history and neuropsychological examination, the patients with mild cognitive impairment underwent
1H-MRS of the brain in three different areas (
Figure 1): left hippocampus, right parietal cortex, and left occipital cortex. Every spectroscopy was performed on a 1.5-T clinical scanner (Signa Horizon, GE Medical Systems, Milwaukee). Sagittal T
1-weighted topogram and T
2-weighted axial localizing series were used to locate volumes (8 cm
3) in cortical areas. Single-voxel
1H-MRS was performed by means of an echo time of 30 msec and a repetition time of 2500 msec with spin-echo technique that uses selective excitation with gradient spoiling for water suppression. The mode of spectral acquisition was Probe-p (PRESS technique).
Data were transferred to a Sun Microsystems workstation and analyzed in an automated manner. Free induction decays were zero filled, exponentially filtered (line broadening=1 Hz), and Fourier transformed. Each spectrum was automatically fitted to four peaks corresponding to levels of N-acetylaspartate (2.02 ppm), total creatine (3.03 ppm), choline-containing compounds (3.23 ppm), and myo-inositol (3.56 ppm). We also calculated the ratios of the peak amplitude of the metabolites relative to creatine and the N-acetylaspartate/myo-inositol ratio in each of the three areas of exploration.
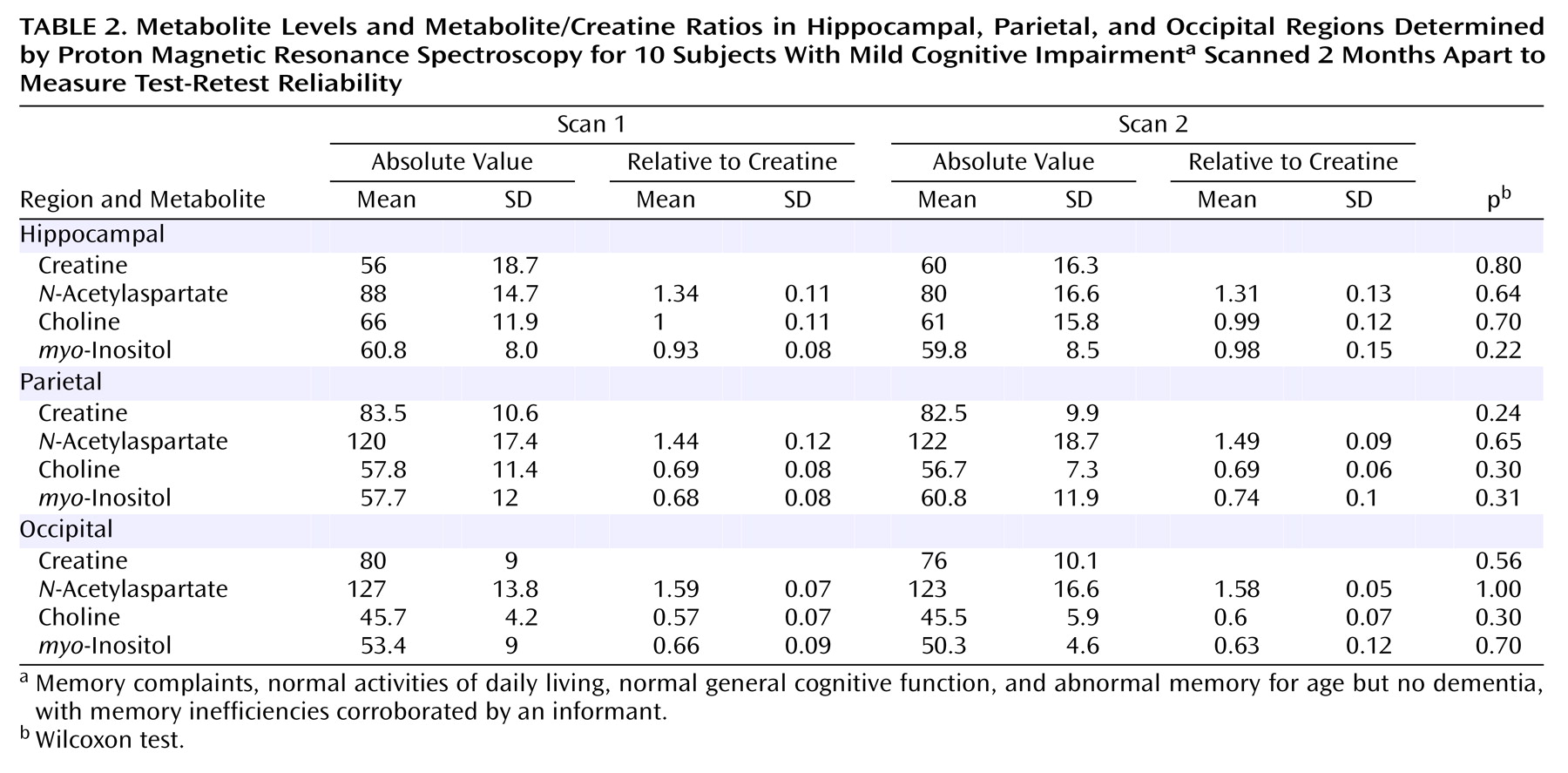
For reproducibility purposes we repeated the exploration after 2 months in 10 patients with the same localizing protocol.
Every patient was followed and reevaluated every 6 months—or earlier if required by caregivers or family physicians—for a mean period of 3 years (range=2.5–3.5 years). Patients with depressive symptoms were adequately treated in the psychiatry unit and reevaluated after 2 months so as to clarify that those patients really met the criteria for mild cognitive impairment. The main end point to be considered was the development of dementia. Neurological evaluations were blinded to the 1H-MRS findings until the end of the follow-up.
Criteria for probable Alzheimer’s disease per the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA)
(26) were applied to the patients who became demented.
The demographic, clinical, and radiological data of patients whose baseline mild cognitive impairment did or did not convert to probable Alzheimer’s disease were analyzed separately and compared statistically. Quantitative variables were compared with t tests for independent samples. The predictive value of the different measurements obtained in baseline 1H-MRS scans was analyzed by means of receiver operating characteristic curves. For this purpose we used the Med-Calc software, which selects the best cutoff for prediction taking into account sensitivity, specificity, likelihood ratio, and predictive values. Complementary statistical analysis was based on a cross-validation technique with the option discriminant analysis/classify/leave-one-out technique of the SPSS, version 10 (SPSS, Inc., Chicago).
Informed consent was obtained from patients and relatives.
Results
At the beginning of the study we had to exclude four patients who did not tolerate the practice of MRI. Therefore, 55 patients were included in the final follow-up assessments. The average age was 72.7 years (SD=5.3, range=61–84). There were 34 women and 21 men. The mean follow-up length was 3 years (range=2.5–3.5), and the clinical assessments were done every 6 months. Each patient was examined six times on average (SD=1).
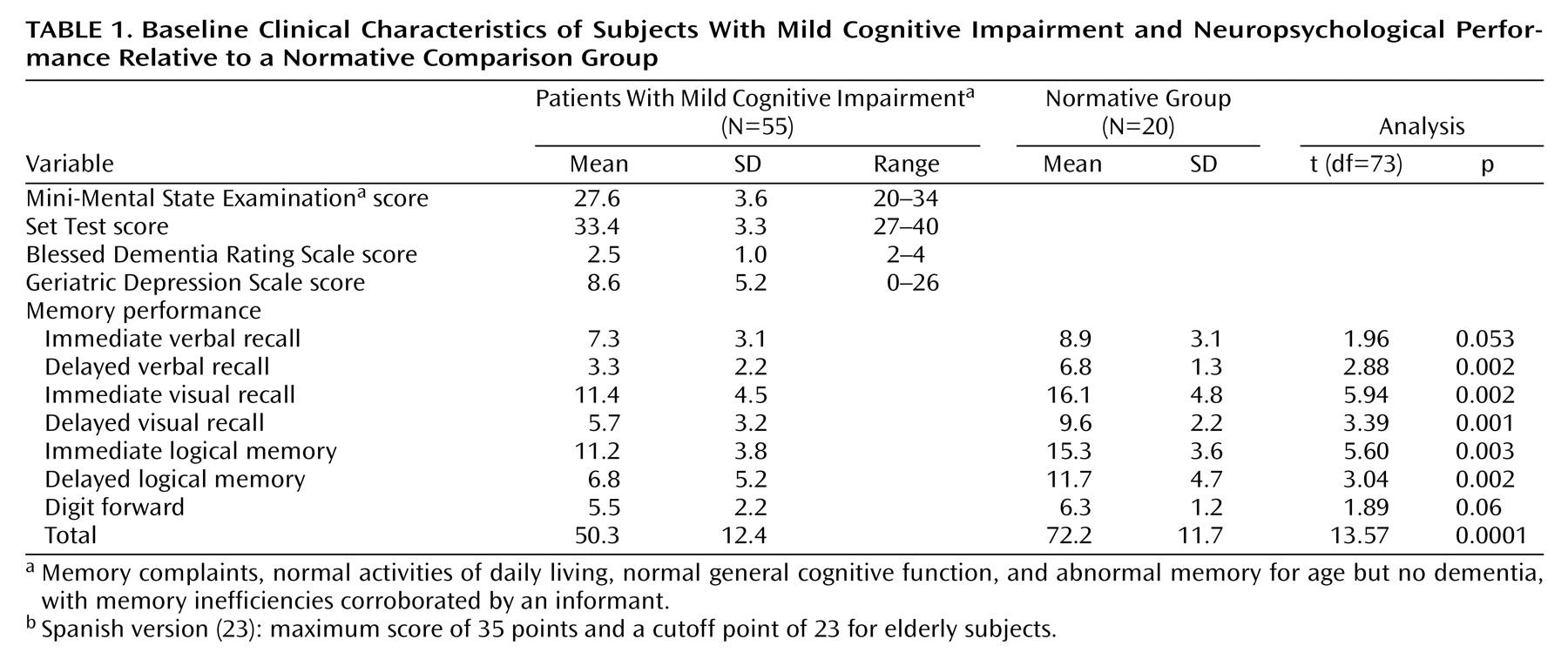
The baseline clinical variables are summarized in
Table 1, including the memory performance of a normative group of 20 healthy subjects (11 women and nine men, mean age=71.4 years). We found significant between-group differences in scores on most memory subscales and total memory score according to two-tailed t tests. All patients with mild cognitive impairment scored 0.5 on the Clinical Dementia Rating and 3 on the Global Deterioration Scale. Six patients scored below 23 on the Spanish version of the MMSE, but this was due to low educational level and not to mental deterioration.
In
Table 2 we present the mean metabolite values and ratios from the first and second scans of 10 patients and found no significant changes. In
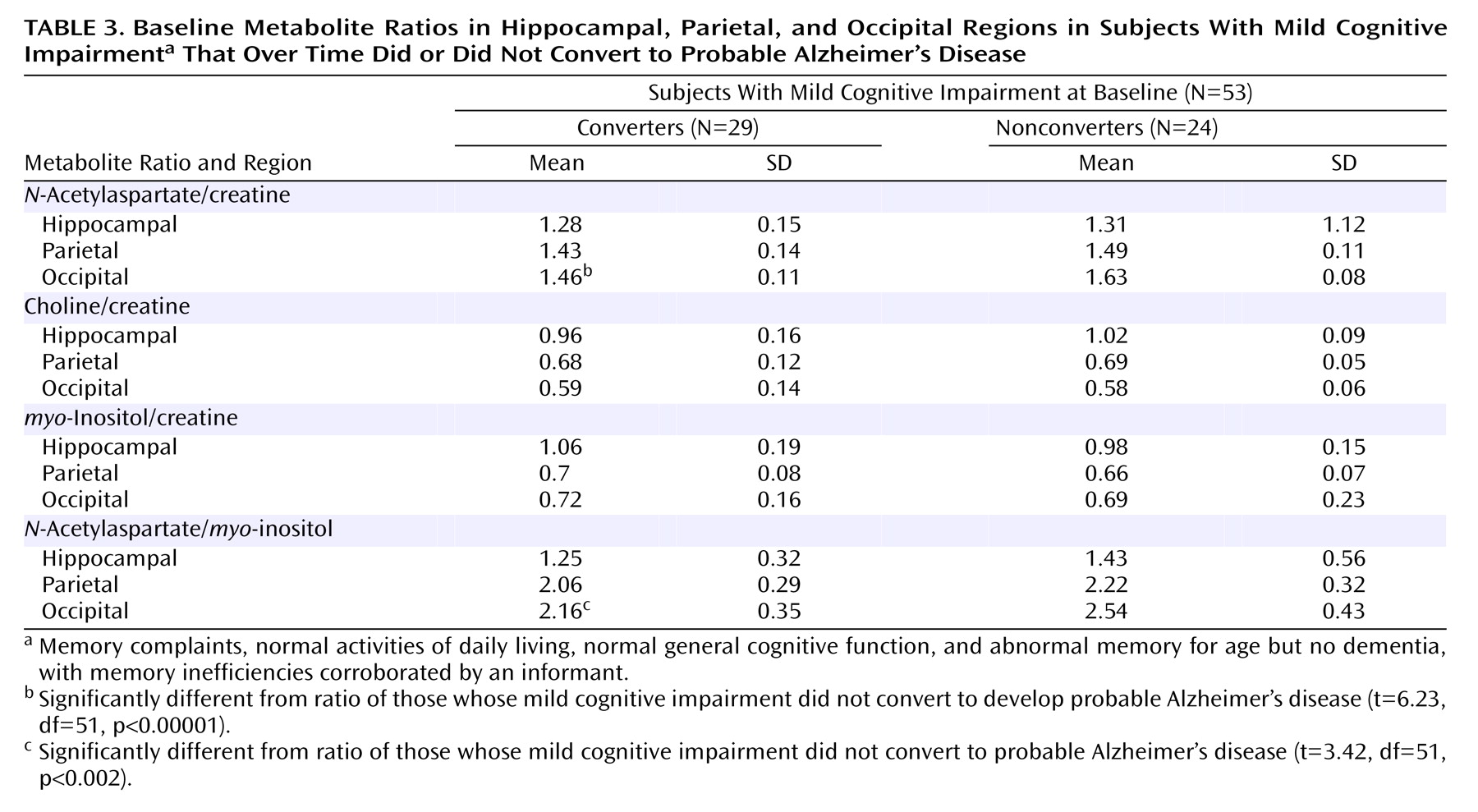
Table 3 are reported the baseline mean values of the different metabolite ratios found in the three areas of exploration. In
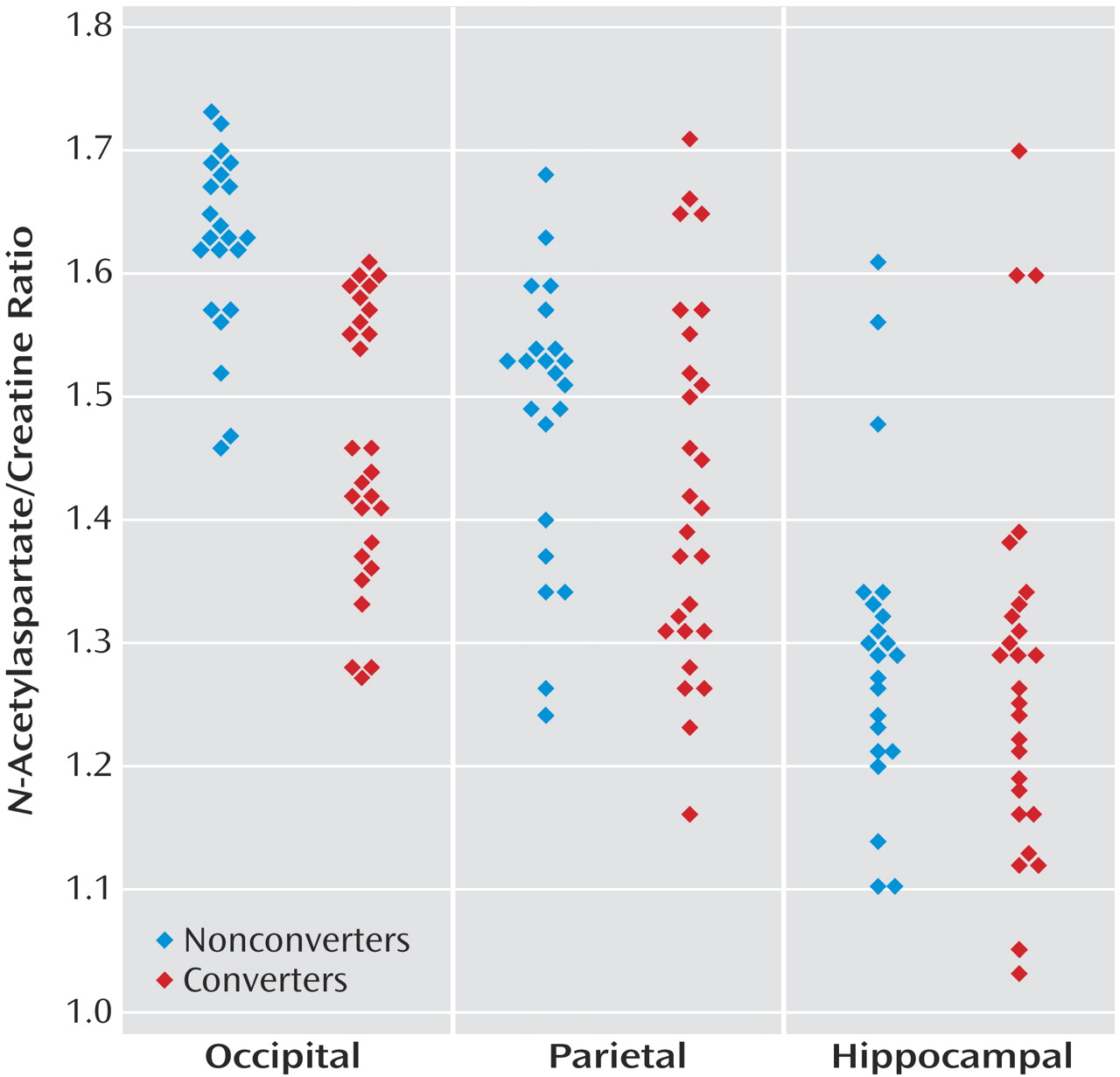
Figure 2, scatterplots representing the
N-acetylaspartate/creatine ratios for the three areas explored are shown.
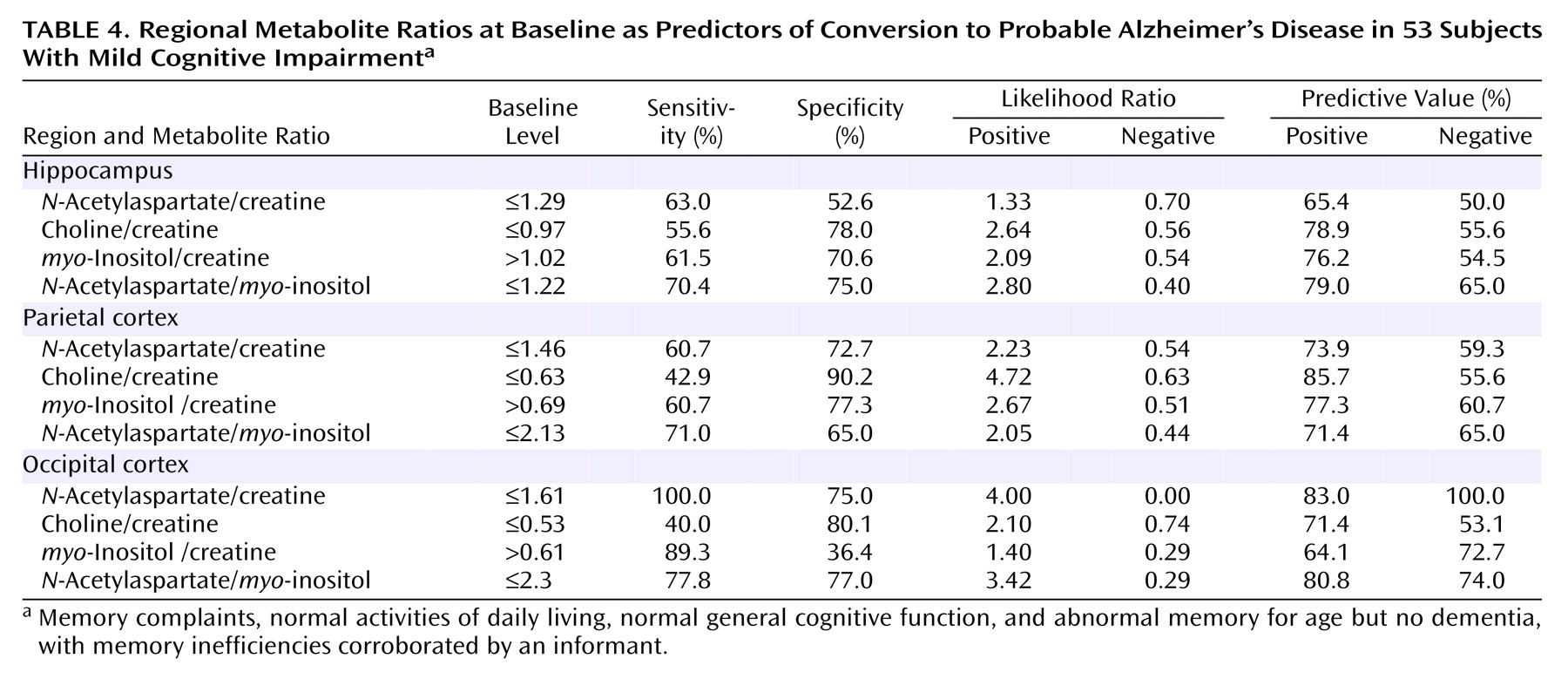
After 1 year, one patient was lost, and another nondemented patient had died from cardioembolic stroke. The remaining 53 patients completed the follow-up period. Of these 53 patients with mild cognitive impairment at baseline, 13 (25%) converted to a diagnosis of probable Alzheimer’s disease per NINCDS-ADRDA criteria. At this stage we found that an occipital cortex N-acetylaspartate/creatine ratio equal to or lower than 1.61 predicted conversion to dementia at 100% sensitivity and 46% specificity; the area under the curve was 0.69 (95% CI=0.55–0.80). In the hippocampus, a myo-inositol/creatine ratio higher than 1.04 predicted conversion at 66.7% sensitivity and 72% specificity, with an area under the curve of 0.66 (95% CI=0.51–0.79). None of the remaining variables predicted conversion to dementia with statistical significance as the value 0.5 was included in the confidence intervals.
After a mean follow-up period of 3 years, 29 (55%) out of 53 patients converted to a probable Alzheimer’s disease diagnosis, and 24 remained with memory loss but did not exhibit dementia. We did not encounter any other type of dementia (Lewy body disease, frontotemporal dementia, or vascular dementia). After this period two patients died after developing dementia. None of the patients reverted to normality from mild cognitive impairment or reverted to mild cognitive impairment from dementia. Treatment for depression in 15 patients barely improved memory performance.
Only the occipital cortex
N-acetylaspartate/creatine ratio at baseline predicted conversion to dementia with confident values (
Table 3 and
Table 4). The mean ratio for demented patients was 1.46 (SD=0.11) and for patients free of dementia it was 1.63 (SD=0.08), a significant difference.
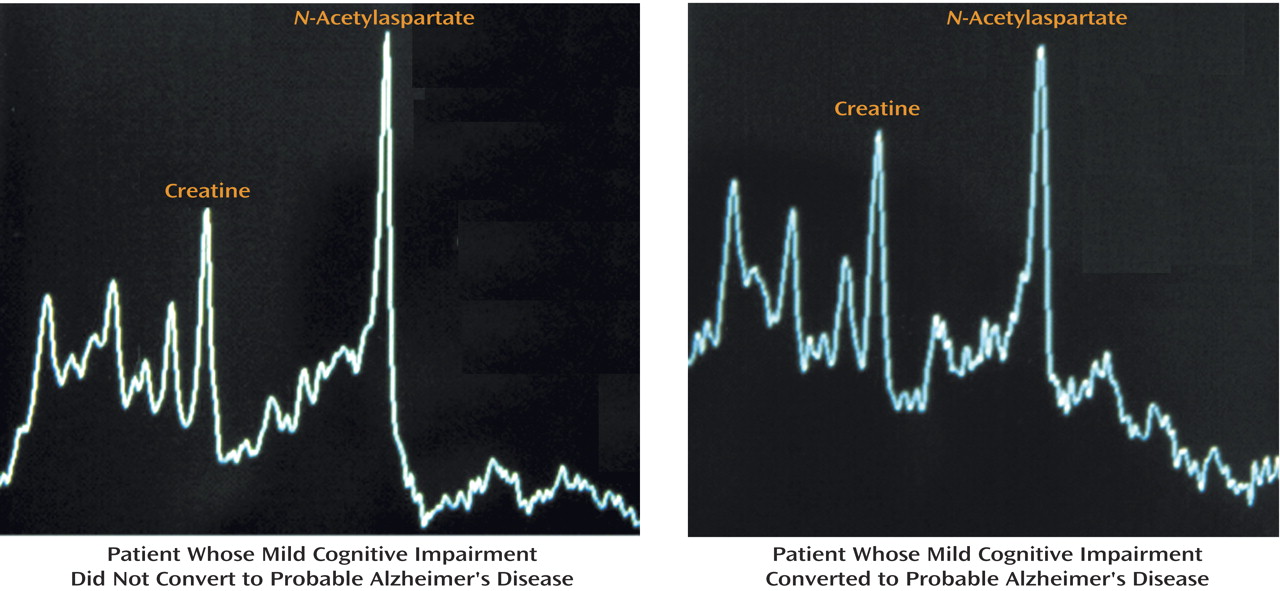
Figure 3 shows spectrum examples with the metabolite peaks for a nonconverter and converter, respectively.
N-Acetylaspartate was significantly decreased in relation to creatine in converters. The mean occipital cortex
myo-inositol/creatine ratio was 0.69 for nonconverters and 0.72 for converters, which was not significant (p=0.50). In the receiver operating characteristic curve analysis, an occipital cortex
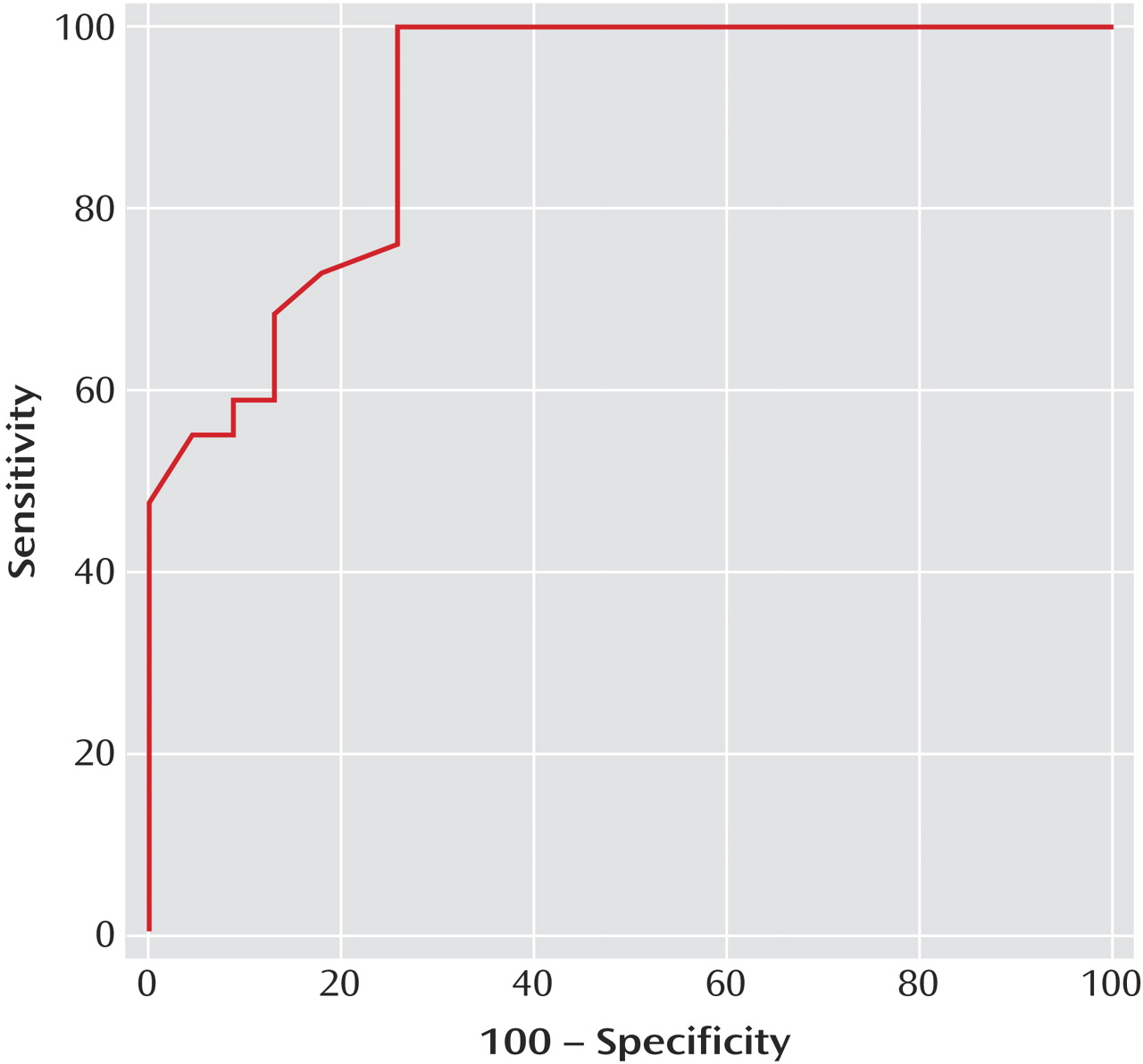
N-acetylaspartate/creatine ratio equal to or lower than 1.61 predicted conversion at 100% sensitivity and 75% (95% CI=53%–90%) specificity. The area under the receiver operating characteristic curve (
Figure 4) was 0.91 (95% CI=0.80–0.97), with a positive predictive value of 83% and a negative predictive value of 100%. The cross-validation analysis yielded a classification accuracy of 88.7% (canonical correlation=0.8, Wilks’s lambda=0.37, χ
2=49.1, p=0.0001). The addition of other variables such as the MMSE score or other metabolite ratios did not improve the model of predictions in the discriminant analysis.
If the cutoff point were ≤1.46 for the N-acetylaspartate/creatine ratio, then specificity would be 95% (95% CI=78%–99%), but sensitivity would decrease to 55% with a positive predictive value of 94% and a negative predictive value of 64%.
The remaining occipital cortex ratios and ratios obtained from the hippocampal and parietal regions did not predict conversion significantly (
Table 3 and
Table 4). A choline/creatine ratio equal to or lower than 0.63 in the right parietal cortex disclosed a 42.9% sensitivity and a 90.2% specificity with a positive predictive value of 85.7%. The absolute metabolite concentrations were not of value for predicting purposes. All predictions were based on baseline
1H-MRS scans.
We also observed that none of the following baseline variables predicted conversion to dementia with confidence: MMSE score, memory performance, verbal fluency, age, or family history of dementia. After 1 year, a score equal to or lower than 26 on the MMSE (Spanish version with a maximum of 35 points) predicted conversion at 61.5% sensitivity and 83.3% specificity, with an area under the curve of 0.71 (95% CI=0.55–0.84). At the 3-year follow-up assessment, the values of prediction for a cutoff point of 29 were as follows: 76.9% sensitivity, 68.2% specificity, and an area under the curve of 0.76 (95% CI=0.60–0.88). The values were below 70% for verbal fluency scores and memory performance scores and, therefore, far from being clinically useful.
Discussion
Memory impairment is a frequent complaint in the elderly, but hitherto we did not have any parameter objective enough to predict conversion to Alzheimer’s disease. Numerous studies have focused on this issue; some of them have used neuropsychological tests as predictors, others have used neuroimaging techniques. Although most studies are interesting, the matter has not been completely resolved because of either insufficient sensitivity or specificity of the tools used.
A cohort of 195 patients with questionable dementia underwent comprehensive neuropsychological examination to predict conversion to dementia, but these predictions achieved at most 64% sensitivity and 76% specificity for verbal recognition and verbal fluency
(27). There have been some reports suggesting that hippocampal volume changes in patients with mild cognitive impairment may help predict conversion to Alzheimer’s disease
(15,
28,
29). However, the subject of which region of the brain, entorhinal cortex or hippocampus, demonstrates the earliest abnormalities in early Alzheimer’s disease has not yet been elucidated because of the large variability of imaging measures, the intersubject variability of brain anatomy, and the imaging artifacts in the medial temporal region
(30). In addition, some degree of hippocampal atrophy may be seen in up to one-third of cognitively normal old people
(31). It is clear that we need more powerful objective parameters, such as magnetic resonance spectroscopy, on which predictions can rely.
Our results are encouraging, but it is surprising to us that hippocampal values did not predict conversion with confidence. It is not consistent with the early involvement of this area in Alzheimer’s disease, but there may be some reasons to explain this finding. First, the partial volume effect in the hippocampus and parietal lobe resulting from the presence of CSF in some patients may affect the absolute value of metabolites but exerts little influence on metabolite ratios, since the CSF virtually does not contain the metabolites being assessed
(32). Given the large size of the voxel, it is probable that extrahippocampal tissues had been included in the analysis, which may decrease the sensitivity of the technique in this area. Another possible explanation is that converters and nonconverters have equal or minimal neuropathological alterations in the hippocampus. A pathoanatomical study that included 13 nondemented cases, four cases with preclinical Alzheimer’s disease, eight cases with very mild symptomatic Alzheimer’s disease, and four cases with severe Alzheimer’s disease found a significant decrease in neuron number in the entorhinal cortex and hippocampus of patients with very mild Alzheimer’s disease but not in healthy and preclinical Alzheimer’s disease cases
(33). The authors concluded that Alzheimer’s disease carries clinical deficits only when it produces significant neuronal loss and recognized that most subjects ascribed to the group of very mild Alzheimer’s disease cases were rated as probable Alzheimer’s disease according to Consortium to Establish a Registry for Alzheimer’s Disease criteria. Theoretically, the lack of significant decrease in neuron number could explain the lack of predictive power of
1H-MRS hippocampal values in our study. Likewise, in a report of 15 patients with mild cognitive impairment who underwent neuropathological examination, all had more neuritic plaques in the hippocampus than did normal subjects, but the plaque density was less than one-third of that found in temporal and insular cortices
(34).
On the one hand the useful values found in the occipital cortex look inconsistent at first glance with the hypothesis that the occipital cortex is only affected in the advanced stages of Alzheimer’s disease
(35). In addition, a cross-sectional study that included 21 patients with mild cognitive impairment, 21 patients with Alzheimer’s disease, and 63 elderly comparison subjects showed similar
N-acetylaspartate/creatine ratios in the occipital lobe (1.78, 1.8, and 1.82, respectively)
(36). However, the authors stated that 14 out of the 21 Alzheimer’s disease patients were being treated with donepezil, which may elevate the
N-acetylaspartate/creatine ratios.
On the other hand, there have been three reports
(37–
39) showing low
N-acetylaspartate/creatine ratios in the midline occipital cortex of Alzheimer’s disease patients (1.14, 1.16, and 1.1, respectively). Furthermore, in one of these studies it was concluded that the occipital cortex
myo-inositol/
N-acetylaspartate ratio had the best discriminant power because it distinguished Alzheimer’s disease from normality at 83% sensitivity and 98% specificity. The authors argued that this cortical area is rich in gray matter and, therefore, ideal for analysis in Alzheimer’s disease
(38).
Our results are also consistent with the delay of the P2 component of visual evoked potential in Alzheimer’s disease patients but not in comparison subjects or patients with other dementing processes
(40), which suggests cholinergic defect in visual association areas. The normal latencies of early visual evoked potential components are compatible with spared primary visual cortex. It was corroborated in a histological study showing low neurofibrillary tangles in primary projection area 17 but increased 20-fold in adjacent association areas 18 and 20, although no differences were seen with regard to number of neuritic plaques
(41).
The exploration of other cortical areas such as the cingulate gyrus also could be of aid in improving predictions. In a cross-sectional study that included 24 patients with mild cognitive impairment and 22 Alzheimer’s disease patients,
1H-MRS of the posterior cingulate gyrus discriminated Alzheimer’s disease from mild cognitive impairment at 80% specificity and 67% sensitivity by determining the
N-acetylaspartate/creatine ratio
(42).
In conclusion, 1H-MRS of the occipital cortex may be a valuable tool in predicting conversion from mild cognitive impairment to probable Alzheimer’s disease and has proven superior to other predictors including neuropsychological performance. Given the correlational and descriptive nature of the present investigation, our findings should be replicated in a larger investigation. If our results are confirmed, this technique could be used for screening or prognostic purposes in patients with mild cognitive impairment. In addition, it could be helpful to determine which patients would benefit from early therapy for Alzheimer’s disease.