Over 33% of women and 20% of men ages 65 and older will develop dementia during their lifetime, and many more will develop a milder form of cognitive impairment
(1). With the aging of the population in many Western countries, the incidence and prevalence of Alzheimer’s disease and other forms of dementia are expected to quadruple over the next 50 years, highlighting the importance of preventive interventions
(2). To our knowledge, no effective preventive therapies have been identified from randomized trials thus far. There is also growing interest in preventing milder cognitive deficits that are not severe enough to meet criteria for the diagnosis of dementia, as patients with this disorder frequently progress to dementia, especially Alzheimer’s disease
(3,
4).
The decline in serum estrogen levels following menopause has been hypothesized to increase the risk of developing cognitive impairment and Alzheimer’s disease in older women. Estrogen has beneficial biological effects in animal and cell culture models that support this hypothesis; these effects include stimulation of choline acetyltransferase activity in ovariectomized rats, neurotrophic effects on cultured neurons, and inhibition of amyloid formation
(5). The results of meta-analyses of the findings from observational studies indicated that postmenopausal estrogen therapy reduces the risk of developing dementia by approximately 30%
(6,
7). However, in recent large randomized trials, not only did combined conjugated estrogen and progestin therapy
(8) or unopposed estrogen
(9) fail to prevent all-cause dementia but there was an increase in dementia associated with hormone assignment.
Raloxifene is a selective estrogen receptor modulator used for prevention and treatment of osteoporosis
(10,
11). Raloxifene has shown estrogen agonist effects on the CNS in animal
(12) and in vitro
(13) studies but anti-estrogen effects as well
(14). We evaluated the effect of raloxifene treatment among older women with osteoporosis to determine if raloxifene alters the risk of developing Alzheimer’s disease and cognitive impairment.
Method
Study Subjects
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial enrolled 7,705 postmenopausal women with osteoporosis. The primary outcomes of the trial were vertebral fractures and bone mineral density. The participants were randomly assigned by site to receive 60 mg/day of raloxifene, 120 mg/day of raloxifene, or identical placebo. All women were also asked to take calcium (500 mg) and vitamin D (400 to 600 IU) daily. Details of the study design and the main results already have been reported
(10,
15). The human studies review board at each site approved the protocol; after complete description of the study to the subjects, written informed consent was obtained.
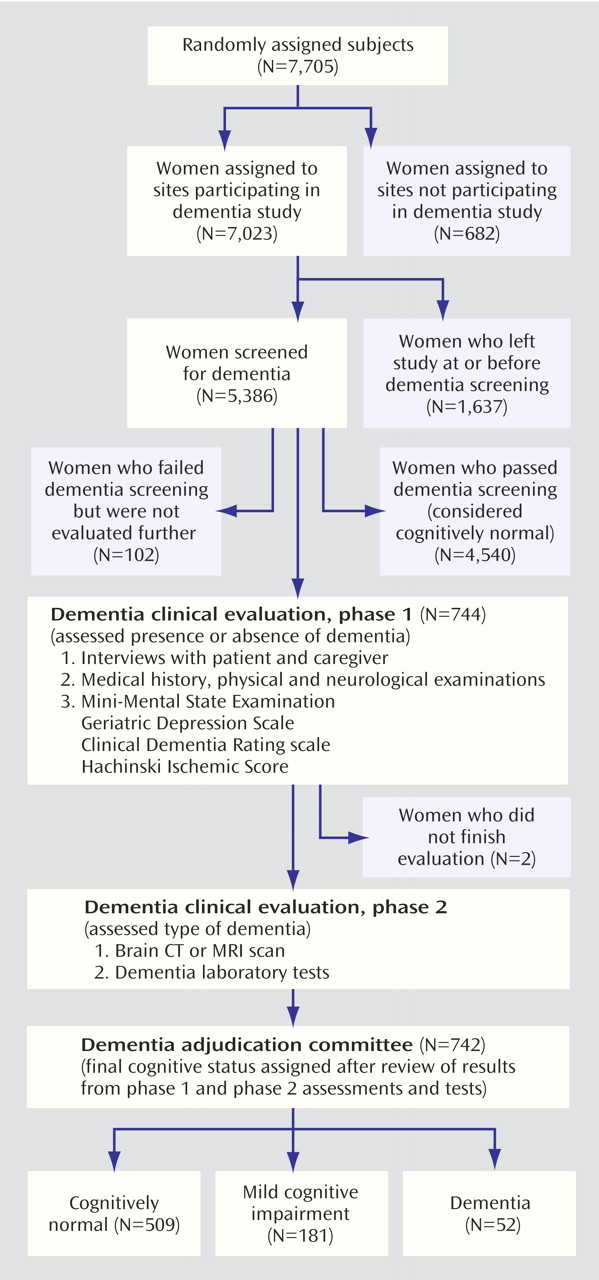
The effect of raloxifene on the occurrence of mild cognitive impairment and dementia was a planned secondary objective of the trial. Of the 180 clinical sites in 25 countries, 19 sites (682 women) were unable or unwilling to participate in the dementia evaluation (
Figure 1). The analysis includes the 5,386 women who were still participating in the MORE study at the time of dementia screening (the end of the third year of treatment).
Measurements
At baseline we collected information on age, ethnicity, education, smoking, alcohol use, health conditions, prior postmenopausal estrogen use (whether the woman had ever used estrogen, up to 2 months before enrollment), and reproductive history. Height and weight were measured, and the body mass index was calculated. The participants completed the 15-item Geriatric Depression Scale
(16), which assesses depressive symptoms over the past week. Total serum estradiol was measured at baseline, after at least a 6-hour fast, in serum shipped the same day to the laboratory (SciCor [now Covance], Central Laboratory Services, Indianapolis). Estradiol concentration was determined by using a double antibody procedure. The intra-assay coefficient of variation is 6.5% (with a standard deviation of 2.1 pmol/liter) at an estradiol concentration of 33 pmol/liter. The detectable limit of the assay used is ≥5 pmol/liter. For our analyses, we dichotomized total estradiol as detectable or nondetectable.
Cognitive Impairment and Dementia
As defined at baseline, the primary outcomes of the dementia study were the risks of developing Alzheimer’s disease, mild cognitive impairment, and dementia of any type. The secondary outcomes were the risk of developing vascular dementia and the risk of any cognitive impairment (mild cognitive impairment and dementia combined).
Screening
Six cognitive tests, similar to those used by the Consortium to Establish a Registry for Alzheimer’s Disease
(17), were administered at baseline and annually in a standard order by trained study personnel who were blinded to treatment assignment. The Short Blessed Test
(18) assesses orientation, concentration, and memory. Scores ranged from 0 to 28, with lower scores indicating better performance, and a score of 8 is consistent with probable cognitive impairment. Trail Making Tests A and B measure visuospatial scanning, sequential processing, motor speed, executive function, and attention
(19). The Word List Memory and Recall Tests measure learning, immediate memory, and delayed memory
(17). The memory test requires immediate recall of 10 standardized words. The Word List Fluency Test measures verbal production, semantic memory, and language by having subjects name as many animals as possible in 60 seconds
(17).
We used a staged evaluation similar to that used in clinical practice to diagnose cognitive impairment and dementia. We used the score on the Short Blessed Test to screen for possible cognitive impairment or dementia. At year 3, participants with the worst 10% of scores in each country or having clinical symptoms of cognitive impairment as judged by the investigator were referred for further evaluation. At two sites, the Short Blessed Test was not administered (N=188). At these two sites, women scoring in the worst 10% on the Buschke Selective Reminding Test (a test of verbal memory)
(20) and those judged impaired by the site investigator were referred for further evaluation. Women who passed dementia screening at year 3 (N=4,540) were considered cognitively normal and were not evaluated further.
Diagnosis
The women who were suspected of having cognitive impairment or dementia were evaluated further by clinical experts to determine the presence of dementia or cognitive impairment and, among those with dementia, to assess the type of dementia (
Figure 1). A blinded clinician who was expert in dementia diagnosis (geriatrician, psychiatrist, or neurologist) 1) interviewed the participant and caregiver for the participant’s history of cognitive deficits, functional abilities, and potential precipitating factors, 2) obtained a list of current and recent medications, 3) performed a physical and neurological examination, and 4) administered the Mini-Mental State Examination (MMSE)
(21), 30-item Geriatric Depression Scale
(22), Clinical Dementia Rating scale
(23), and Hachinski Ischemic Score
(24). The Clinical Dementia Rating is a semistructured interview of both participant and caregiver that evaluates functional and cognitive status. A summary score (total box score) is calculated from the six individual category scores, and a standardized algorithm is used to assign an overall global Clinical Dementia Rating score. A global score of 1 or higher suggests the diagnosis of dementia, a score of 0.5 suggests mild cognitive impairment, and a score of 0 is consistent with a normal cognitive status
(23).
Each participant with evidence of dementia based on the clinical impression of the consulting clinician or with an MMSE score less than 24 was given a brain scan with computerized tomography or magnetic resonance imaging and laboratory tests (fluorescent treponemal antibody, vitamin B12, serum folate, and thyroid-stimulating hormone). All brain scans were read locally for safety purposes but were subsequently read by a neuroradiologist at the University of California, San Francisco. This central reader, blind to treatment groups, evaluated the scans for abnormal findings and determined if these were clinically relevant.
All results of the medical history, physical examination, functional and mental status assessments, cognitive functioning tests, and brain imaging tests were recorded on standardized forms. These forms, along with laboratory test results, were presented to two members of the dementia adjudication committee. This committee comprised four physicians (including V.W.H.) who are recognized experts in the clinical and research diagnosis of cognitive impairment and dementia. The committee members were masked to treatment assignment. Using criteria established at baseline, two committee members independently judged cognitive status as normal cognitive functioning, mild cognitive impairment
(3,
25), Alzheimer’s disease (based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association
[26]), vascular dementia (based on the criteria of the State of California Alzheimer’s Disease Diagnostic and Treatment Centers
[27]), or other type of dementia. If the committee confirmed dementia but could not assign a type, the diagnosis of “dementia type indeterminate” was assigned.
Statistical Analyses
We compared baseline characteristics by treatment group, using chi-square tests for categorical data, analysis of variance (ANOVA) for normally distributed continuous variables, and Kruskal-Wallis tests for skewed continuous variables. Baseline characteristics were analyzed for the 5,386 women who participated in the dementia ancillary study. Among the 742 women who completed phase 1 and phase 2, we also compared baseline characteristics by final diagnosis.
We determined the incidence of each cognitive diagnosis by treatment group and calculated the relative risk and 95% confidence intervals (CIs) by using log-linear regression models for comparisons of the 60- and 120-mg/day doses of raloxifene with placebo (intention-to-treat analyses). All women who passed the dementia screening (N=4,540) were considered to be cognitively normal, and we adjusted the models by country to control for variation in cognitive test scores. The 102 women who should have been clinically evaluated but did not participate (
Figure 1) and the two women who did not complete the evaluation were coded as missing. We repeated the analyses after coding them as having any cognitive impairment to determine if our results were sensitive to this categorization. We anticipated having only moderate power to detect differences in dementia outcomes by treatment group, given the low incidence rates of dementia and mild cognitive impairment.
We examined the possibility of pooling the data from the two raloxifene dose groups by performing a chi-square test on the incidence rates of the cognitive outcomes. Since these rates were statistically different for some diagnoses, we analyzed the data separately by dose of raloxifene.
To examine the effect of baseline serum total estradiol level on the association between raloxifene and cognitive outcome (a post hoc analysis), we stratified the women by whether or not serum estradiol was detectable at baseline. We also tested for the possibility of an interaction between treatment group and estradiol level for all cognitive outcomes.
Results
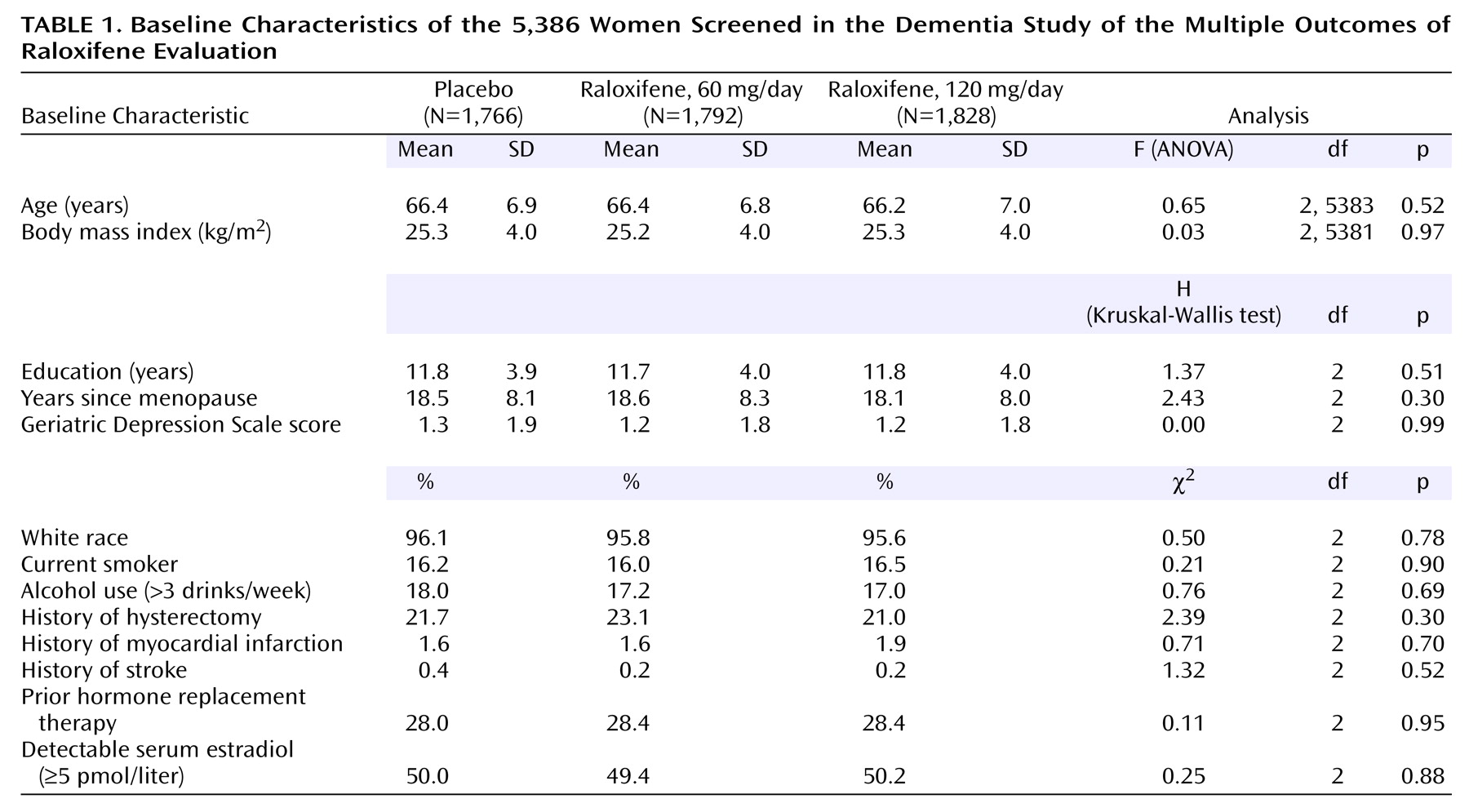
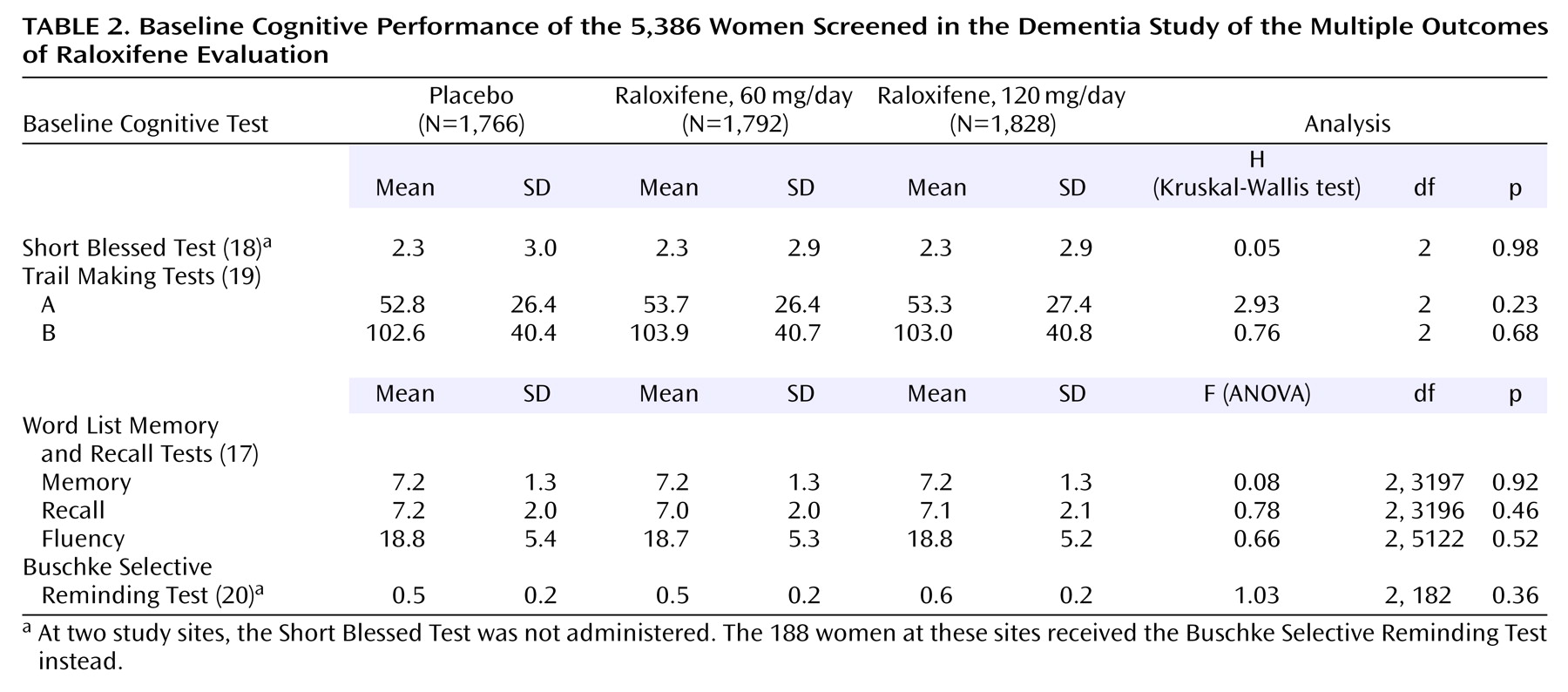
There were no significant differences between treatment groups in baseline characteristics (
Table 1) or baseline cognitive test scores (
Table 2). The mean age of the participants was 66.3 years with a range of 35.7 to 80.9 years. Of the 5,386 participants, 744 women had suspected dementia at screening and were evaluated further (
Figure 1). An additional 102 women were suspected of having dementia but were not evaluated further. The 102 women suspected of dementia who did not undergo further evaluation were equally distributed among the three treatment groups (p=0.64, chi-square analysis). The 744 women who were suspected of having dementia were generally older (p<0.001, ANOVA), further past menopause, less educated, and more depressed at baseline than were the women not referred for further clinical evaluation (p<0.04, Kruskal-Wallis test), and they were also more likely to have had a hysterectomy and less likely to drink alcohol at baseline (p<0.04, chi-square analysis). The mean time between randomization and the completion of the clinical dementia evaluation was 3.8 years and did not differ by treatment group.
We determined if the 5,386 women included in our analyses were similar to the entire 7,705 women who began the MORE trial and found that on almost all of the baseline characteristics, there were no statistically significant differences between these groups. In addition, similar rates of women in the three treatment groups were referred for the clinical dementia evaluation, and there were no statistically significant differences in baseline characteristics between the treatment and placebo groups. Among the 5,386 women who were part of the dementia evaluation study, the rate of compliance (taking 70% or more of the study drug between visits) was 94.2% for placebo, 94.6% for 60 mg/day of raloxifene, and 94.6% for 120 mg/day of raloxifene. These rates were not significantly different (p=0.54, chi-square analysis).
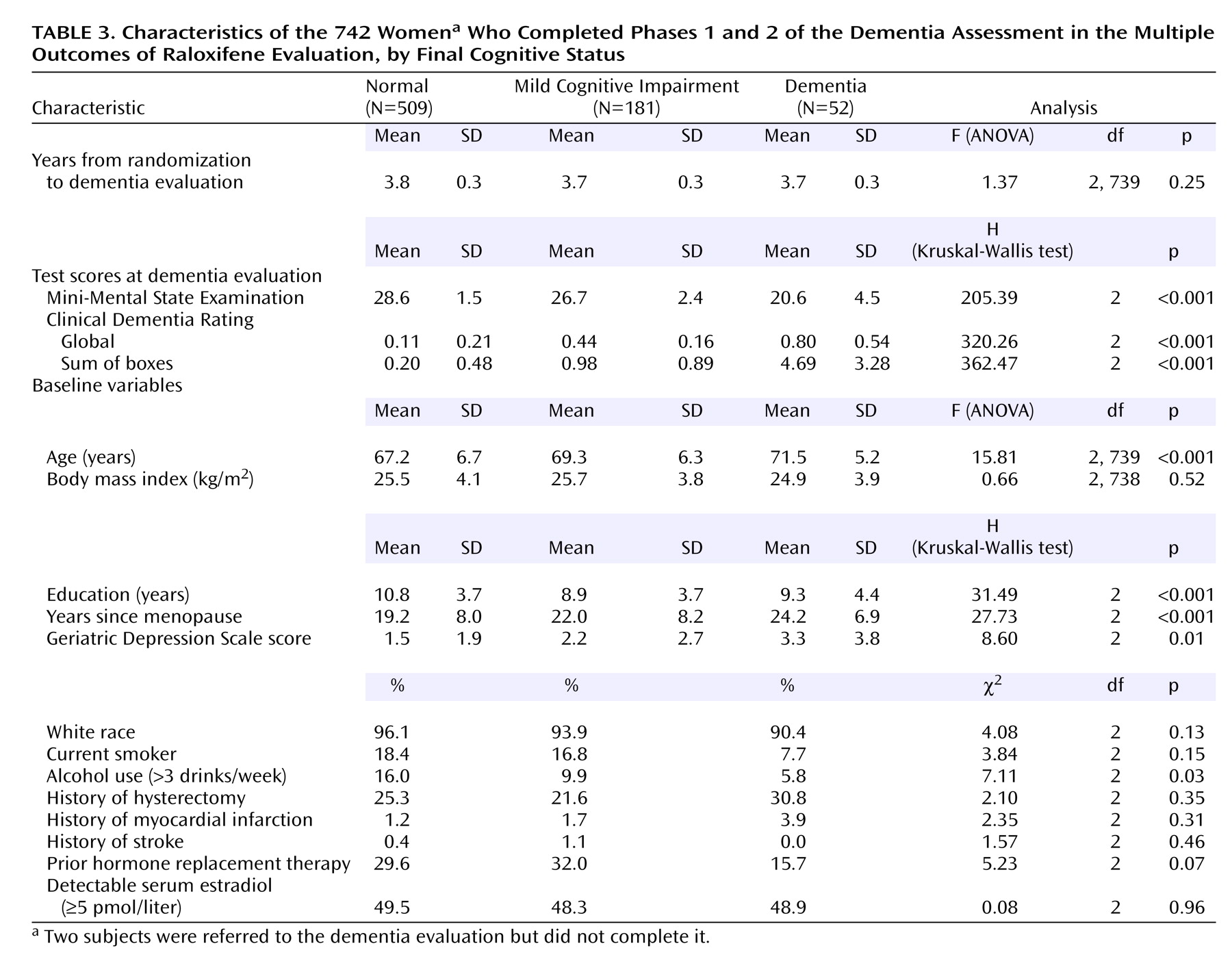
Of the 744 women referred for further evaluation of dementia, 742 completed the evaluation. Of these, 509 (68.6%) were found to have normal cognitive functioning, 181 (24.4%) had mild cognitive impairment, and 52 (7.0%) met the criteria for dementia (36 with Alzheimer’s disease, one with vascular dementia, and 15 with indeterminate or other type of dementia). As expected, women with mild cognitive impairment or dementia were progressively older, were further past menopause, were less educated, had higher depression scores, and were less likely to use alcohol than those with normal cognitive functioning (
Table 3). The percentage of women with a history of estrogen use was nonsignificantly lower for women who developed dementia than for those who developed mild cognitive impairment or who were cognitively normal. Mean scores on the MMSE and Clinical Dementia Rating obtained at the dementia evaluation were worse in women diagnosed with dementia than in those with mild cognitive impairment or normal cognitive functioning, as expected (
Table 3).
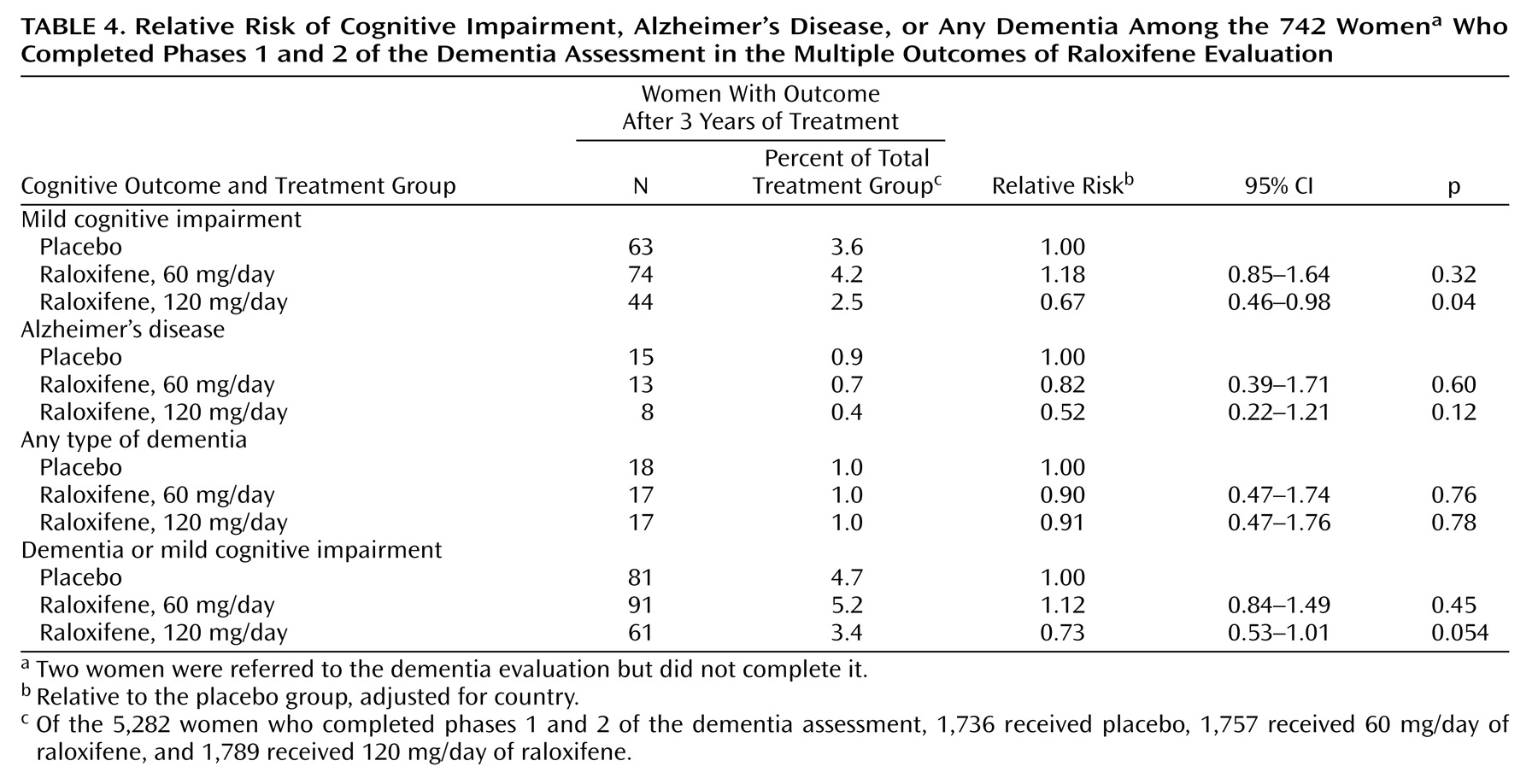
There was no significant difference between the placebo group and the women receiving 60 mg/day of raloxifene in the risk of developing mild cognitive impairment, Alzheimer’s disease, dementia from any cause, or any cognitive impairment (dementia and mild cognitive impairment combined) (
Table 4). Compared with the placebo group, women receiving 120 mg/day of raloxifene had a significantly lower risk of developing mild cognitive impairment (33% lower), a nonsignificantly lower risk of developing Alzheimer’s disease, and a nearly significantly lower risk of developing any cognitive impairment. The risk of dementia from any cause did not differ significantly between the group receiving 120 mg/day of raloxifene and the women taking placebo. When we repeated our analyses by categorizing the 104 women who had a high likelihood of cognitive impairment but did not start or complete the dementia evaluation as having any cognitive impairment, our results were similar.
The effects of raloxifene 60 and 120 mg/day of raloxifene on the risk for mild cognitive impairment and any cognitive impairment were significantly different (p=0.002 for mild cognitive impairment and p=0.007 for any cognitive impairment), indicating that the results of the two dose groups cannot be pooled.
Women taking 120 mg/day of raloxifene who had undetectable serum estradiol levels at baseline had a slightly lower relative risk for each cognitive outcome (compared to the placebo group) than women with detectable estradiol levels. For example, the risk for any cognitive impairment among those who were assigned to 120 mg/day of raloxifene and had undetectable estradiol levels was 0.62 (95% CI=0.37–1.04) and for those with detectable levels the risk was 0.84 (95% CI=0.50–1.39), but these interactions were not statistically significant (p>0.34 for all interactions).
Discussion
Raloxifene, at a dose of 120 mg/day, reduced the risk of developing mild cognitive impairment by 33% among postmenopausal women with osteoporosis. In the MORE trial, one case of mild cognitive impairment was prevented for every 91 women treated. There was also a somewhat lower risk of developing Alzheimer’s disease among the women treated with the higher dose of raloxifene that did not reach statistical significance. Given that few women developed Alzheimer’s disease, the lower risk observed among women assigned to 120 mg/day of raloxifene may have occurred by chance. However, most people with mild cognitive impairment progress to develop dementia over several years, and the majority of these develop Alzheimer’s disease
(3,
4). Thus, a drug that lowers the risk of mild cognitive impairment might also be expected to reduce the risk of developing Alzheimer’s disease. As our results are tentative, trials enrolling women at high risk for cognitive impairment or Alzheimer’s disease, or longer trials, are required to establish the effect of treatment with raloxifene on the risk for developing these cognitive outcomes.
We observed a differential effect of raloxifene on cognitive outcomes by dose. While treatment with 120 mg/day of raloxifene reduced the risk of mild cognitive impairment, there was no significant benefit from treatment with the lower dose of 60 mg/day, and the difference in the effects of the two doses was statistically significant. For treatment of osteoporosis, 60 and 120 mg/day of raloxifene appear to be equally effective
(10). However, it is possible that higher doses of raloxifene are needed to cross the blood-brain barrier and produce CNS benefits. Furthermore, the raloxifene dose producing maximal increases in hippocampal choline acetyltransferase activity (3 mg/kg)
(12) is greater than that producing maximal effects on bone (1 mg/kg) in ovariectomized rats
(28).
From the data collected in our trial, we are unable to determine if raloxifene has an estrogenic, anti-estrogenic, or unique effect on the brain. However, given that several observational studies have suggested that estrogen use reduces the risk of developing Alzheimer’s disease or cognitive impairment
(29–
32), it is most likely that raloxifene has an estrogenic effect in the CNS
(33). In rodent models, raloxifene has demonstrated estrogen agonist effects on neurons, including stimulation of neurite outgrowth from cultured cells
(13), stimulation of choline acetyltransferase activity in the hippocampus
(12), and increased 5-hydroxytryptamine-2A receptors in the cingulate and frontal cortices of ovariectomized rats
(34). All of these mechanisms could potentially reduce risk of cognitive impairment. On the other hand, given the recent results from the Women’s Health Initiative trial indicating an increased risk of developing dementia with either estrogen and progestin
(8) or estrogen alone
(9), it is possible that the reduction in risk of cognitive impairment with raloxifene is due to an anti-estrogen effect. Another possible explanation for the differences between our results and those of the Women’s Health Initiative Memory Study may be related to cardiovascular disease. Unlike estrogen
(8,
9), raloxifene is not associated with an increase in stroke and other cardiovascular outcomes that may mediate cognitive impairment
(35). It is interesting that the women with undetectable baseline estradiol levels had a greater reduction in risk associated with 120 mg/day of raloxifene than did the women with higher baseline estradiol levels, although this interaction was not statistically significant.
We previously studied the effect of 3 years of raloxifene treatment, in both the 60 and 120 mg/day groups, on cognitive functioning in women enrolled in the MORE study and reported that there were no differences on mean 3-year scores between treatment groups
(36). However, compared to the women assigned to placebo, those assigned to raloxifene had less risk of developing impairment (as assessed by a cutoff in the change in scores) in verbal memory and attention, cognitive domains affected earliest in mild cognitive impairment and Alzheimer’s disease
(37). In addition, among the women who were at risk for cognitive impairment because they were over 70 years of age, those assigned to raloxifene had smaller 3-year declines in memory and attention. Data from a small, randomized trial of 120 mg/day of raloxifene for women with Alzheimer’s disease also indicated improvement in verbal memory in the raloxifene group after 12 weeks of treatment
(38).
Our trial has several limitations. Most of the women enrolled in the MORE study were white, and all had osteoporosis. We cannot be sure that our results apply to women without osteoporosis, to nonwhite women, or to men. Even though the trial was large, the rate of developing dementia is low until advanced old age, and we had limited power to detect differences in the risk of developing Alzheimer’s disease and other types of dementia. Assuming a two-tailed alpha of 0.05 and 80% power, we were able to detect relative risks of 0.22 or less for Alzheimer’s disease, 0.57 or less for mild cognitive impairment, and 0.61 or less for any cognitive impairment. The observed incidence of 1% per year for the development of any cognitive impairment among the MORE participants is at the low end of reported incidence rates for women ages 65–69 years
(39). These lower rates are not surprising given that all women who enrolled in the MORE study had high levels of functioning at baseline and volunteered for a long-term trial. Our observed mean MMSE and Clinical Dementia Rating scores for women in each diagnostic group are consistent with scores previously reported for these diagnoses
(40,
41), supporting the validity of the criteria used in the dementia evaluation. However, given that some women may have had very mild cognitive deficits at baseline, we cannot determine whether raloxifene (120 mg/day) prevented cognitive impairment or delayed its progression. Finally, some women who were referred for the clinical dementia evaluation did not go or did not complete the evaluation. The number of women in this group did not differ by treatment group, and when we repeated our analyses and coded these women as having cognitive impairment, our results were similar.
Cognitive impairment and especially dementia are devastating conditions that cause severe debility and death among older persons. Thus far, we know of no intervention that has been proven to reduce the risk of developing these conditions directly. Raloxifene at a dose of 120 mg/day (but not 60 mg/day) resulted in a reduced risk of mild cognitive impairment and a nearly significant reduction in any cognitive impairment (mild cognitive impairment or dementia) in postmenopausal osteoporotic women. Additional trials of raloxifene and other selective estrogen receptor modulators for prevention of cognitive impairment, especially in women at high risk, should be conducted to confirm these results.