Mood disorders affect up to twice as many women as men and often emerge during the childbearing years
(1) . Depressive symptoms are not uncommon during pregnancy, and, as suggested in a large cohort study by Evans et al.
(2), symptoms may occur more frequently during pregnancy than in the postpartum period. Antenatal depression is not necessarily benign; it has been associated with low maternal weight gain, increased frequency of cigarette, alcohol, and substance use
(3), and ambivalence about the pregnancy
(4) .
Although consensus guidelines have been developed to educate clinicians and patients about the relative merits of treatment options, the optimal way to treat depression during pregnancy remains a subject of debate
(5,
6) . The safety of antidepressant use during pregnancy has been questioned because medications can potentially have teratogenic effects
(7) . However, two studies
(8,
9) have shown that women on antidepressants who discontinue their antidepressant or decrease the dose during pregnancy to minimize fetal exposure to medications are at risk of a relapse of depression.
The impact on neonatal outcome of exposure to antidepressants during pregnancy is understudied. The potential for problems such as preterm birth, low birth weight, and poor neonatal adaptation shortly after delivery is an issue of concern
(7) . Preterm birth, defined as less than 37 completed weeks of gestation, affects a significant number of births in the United States and is a leading cause of perinatal morbidity
(10) . Potential consequences of preterm birth can include neonatal asphyxia, meconium aspiration, hypoglycemia, polycythemia, respiratory distress syndrome, cerebral palsy, respiratory difficulty, learning disabilities, behavioral problems, and attention deficit hyperactivity disorder
(11) . Despite advances in medicine, rates of preterm birth are higher in the United States than in other developed countries.
A number of studies have suggested that in utero exposure to antidepressants has a negative impact on gestational age at birth
(12 –
19) . Other studies of obstetrical and infant outcome in which gestational age at birth was reported did not find that duration of pregnancy was affected by antidepressant use
(20 –
31) . These negative studies are limited by small sample sizes
(20,
22), antidepressant exposure for only the first trimester or for a limited portion of pregnancy
(23,
26,
27), lack of a comparison group
(23), and lack of control for maternal mood state
(22,
23,
26,
27,
30) . Moreover, some of these studies were not intended or designed to investigate gestational age at birth or preterm birth as a primary outcome measure
(21,
24,
25,
28,
31) and thus may not have used subjects or sample sizes appropriate for examining these variables.
Our study was designed to prospectively follow healthy women across pregnancy, collecting detailed information about symptoms of depression and anxiety and medication use to differentiate the effects of depressive symptoms versus antidepressant use on gestational age at birth and risk of preterm birth.
Method
This study was conducted at the University of California at Los Angeles Semel Institute for Neuroscience and Human Behavior. Written informed consent was obtained from all participants, and the study was approved by the UCLA institutional review board. A total of 122 women were enrolled between July 2000 and November 2005. Participants were followed naturalistically over the course of their pregnancy.
Participants were divided into three groups: group 1 comprised women with major depressive disorder who took antidepressant medication for more than 50% of their pregnancy; group 2 comprised women with major depressive disorder who elected not to take antidepressants during their pregnancy, discontinued antidepressants in the first trimester, and/or had a brief antidepressant exposure (<10 days); and group 3 comprised healthy comparison women with no psychiatric history. Women with major depressive disorder (groups 1 and 2) were recruited from the UCLA Women’s Life Center clinic; the UCLA outpatient obstetric clinics; self-referral from advertisements and community outreach detailing the study; direct referral from community obstetrical practices; and direct referrals from community psychiatric practices. Women were eligible for groups 1 and 2 if they were between the ages of 18 and 45, were in the first trimester of pregnancy, and had major depressive disorder. Exclusion criteria included being actively suicidal, meeting DSM-IV criteria for another current axis I disorder, having a positive urine drug screen, and using medications known to adversely affect the fetus.
Comparison subjects (group 3) were recruited from the UCLA outpatient obstetric clinics and by self-referral from advertisements and community outreach describing the study. Participants were eligible for group 3 if they were between the ages of 18 and 45, were in the first trimester of pregnancy, had no psychiatric history, and did not meet criteria for a current DSM-IV axis I disorder. Exclusion criteria included a positive urine drug screen and the use of medications known to adversely affect the fetus.
A study physician assessed participants at baseline to determine eligibility. At study entry, all participants underwent a Structured Clinical Interview for DSM-IV (SCID)
(32) . Physical data, such as height and prepregnancy weight, and a urine toxicology screen were obtained. Participants underwent assessments on a monthly basis throughout their pregnancy. At each visit, the 28-item Hamilton Depression Rating Scale (HAM-D)
(33) and the SCID mood module for depression were administered by a research assistant blind to diagnosis and treatment. Participants were also seen by the study physician for a clinical assessment, which included collection of information about medications taken, medication dosages, and use of alcohol, cigarettes, and other substances. At each visit, participants also completed the Beck Depression Inventory
(34) and the Perceived Stress Scale
(35), and their body weight was measured.
Primary outcome variables included gestational age at birth, birth weight, 1- and 5-minute Apgar scores, and admission to the special care nursery. Obstetrical and hospital records were obtained for all participants. Gestational age was determined from last menstrual period and verified by early ultrasound examination.
The likelihood ratio chi-square test was used for between-groups comparisons of categorical outcome variables, and analysis of covariance (ANCOVA) was used for continuous outcome variables. When warranted, analyses were followed up with post hoc tests using the Bonferroni correction for multiple testing. The combined effects of the continuous measures for depression (percentage of visits where the patient was positive for major depression according to the SCID) and antidepressant use (percentage of visits the patient was taking antidepressant medication) were analyzed with a hierarchical regression analysis. The relative contributions of different predictor variables were evaluated on the basis of sequential R 2 difference tests.
Results
Of the 122 participants in all three study groups, 93 women completed the study, 12 chose to discontinue participation in the study, one was discontinued because of lack of compliance with the study protocol, eight miscarried, one electively terminated the pregnancy, and seven were lost to follow-up prior to completion of the pregnancy. Of the 93 women who completed the study, 73 had a diagnosis of major depressive disorder. Of these, 49 were in group 1 (women who were on antidepressants for more than 50% of their pregnancy) and 22 were in group 2 (women who elected not to take antidepressants during their pregnancy, discontinued antidepressants in the first trimester, or had a brief exposure to antidepressants). Two women with major depressive disorder who started antidepressants in the final month of pregnancy were excluded from the analyses. Of 20 participants with no psychiatric history, one took fluoxetine during pregnancy as prophylaxis for migraine headaches and was therefore excluded. The remaining 19 women were in group 3 (comparison group).
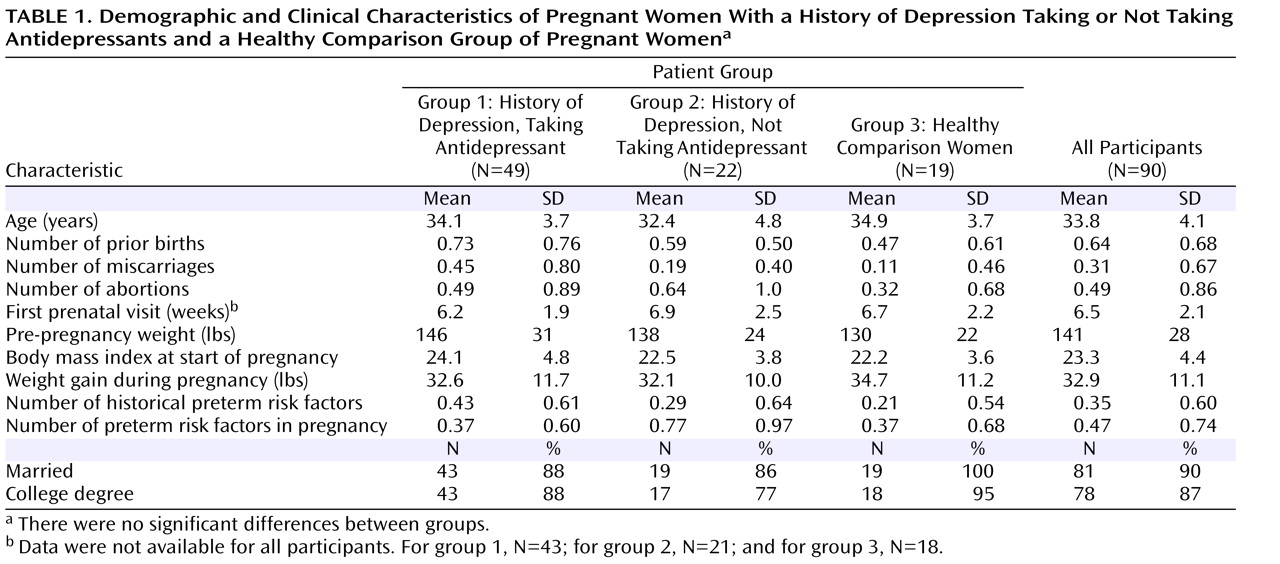
Analyzable data were available for 90 women who completed the study. Demographic and clinical characteristics (
Table 1 ) did not differ significantly between groups. Maternal age, number of primigravidas, number of prior births, body mass index at the start of pregnancy, and maternal weight gain during pregnancy did not differ significantly between groups. Rates of historical preterm risk factors (history of abortions, miscarriages, hypertension, preterm labor, fetal demise, uterine cervical abnormality, myomectomy, renal disease, or pelvic inflammatory disease) and risk factors that developed during pregnancy (anemia, short cervix, asthma, vaginosis, vaginal bleeding, or threatened abortion) were low for all three groups and did not differ significantly between groups. Rates of hypertension or pregnancy-induced hypertension (two in group 1 and one each in groups 2 and 3) and preeclampsia (one each in groups 1 and 2) were low and did not differ significantly between groups.
Substance use was uncommon among participants. One woman in group 1 and one woman in group 3 reported smoking a pack a day of cigarettes during the first 8 weeks and first 5 weeks of pregnancy, respectively. One woman in group 2 and one in group 3 reported having more than one alcoholic drink per week after learning of the pregnancy. Two women in group 1 reported marijuana use early in pregnancy (on one and two occasions, respectively), and one woman in group 2 reported use of marijuana and cocaine on one occasion during early pregnancy.
The antidepressants used by women in group 1 were sertraline, fluoxetine, paroxetine, citalopram, escitalopram, nefazodone, venlafaxine, bupropion, and nortriptyline. Single antidepressant exposures included sertraline (N=15), fluoxetine (N=13), citalopram (N=4), paroxetine (N=4), venlafaxine (N=2), and nortriptyline (N=1). Sequential monotherapy exposures included citalopram and fluoxetine (N=1); paroxetine and sertraline (N=1); escitalopram and citalopram (N=1); nefazodone and fluoxetine (N=1); venlafaxine and sertraline (N=1); fluoxetine, citalopram, and sertraline (N=1), citalopram, fluoxetine, and sertraline (N=1); and venlafaxine, fluoxetine, and sertraline (N=1). Two women were treated with more than one antidepressant at a time (sertraline, venlafaxine, and bupropion [N=1]; and venlafaxine and nefazodone followed by sertraline [N=1]). Forty-four of the group 1 participants took antidepressants in the first trimester, and all 49 took antidepressants in the second and third trimesters. Twenty-five participants had an increase in antidepressant dose at some point during the pregnancy. Many women in group 1 initially decreased their antidepressant dose or switched to a different medication. Some were able to manage for much of the pregnancy on a lower dose and then increased their dose toward the end of the pregnancy because of concern about the risk of postpartum depression. Others were stable on one antidepressant at conception and switched to another antidepressant, which was then increased to a therapeutic dose over the course of the pregnancy. Thus, in this study, increases in antidepressant dose were not necessarily related to worsening depression.
Of the group 2 participants, six took antidepressants only during the first trimester (sertraline [N=3], venlafaxine [N=2], fluoxetine [N=1]), one took citalopram for 6 weeks during the first trimester and 9 days during the second, and one took sertraline for 7 days only in the second trimester.
Depression
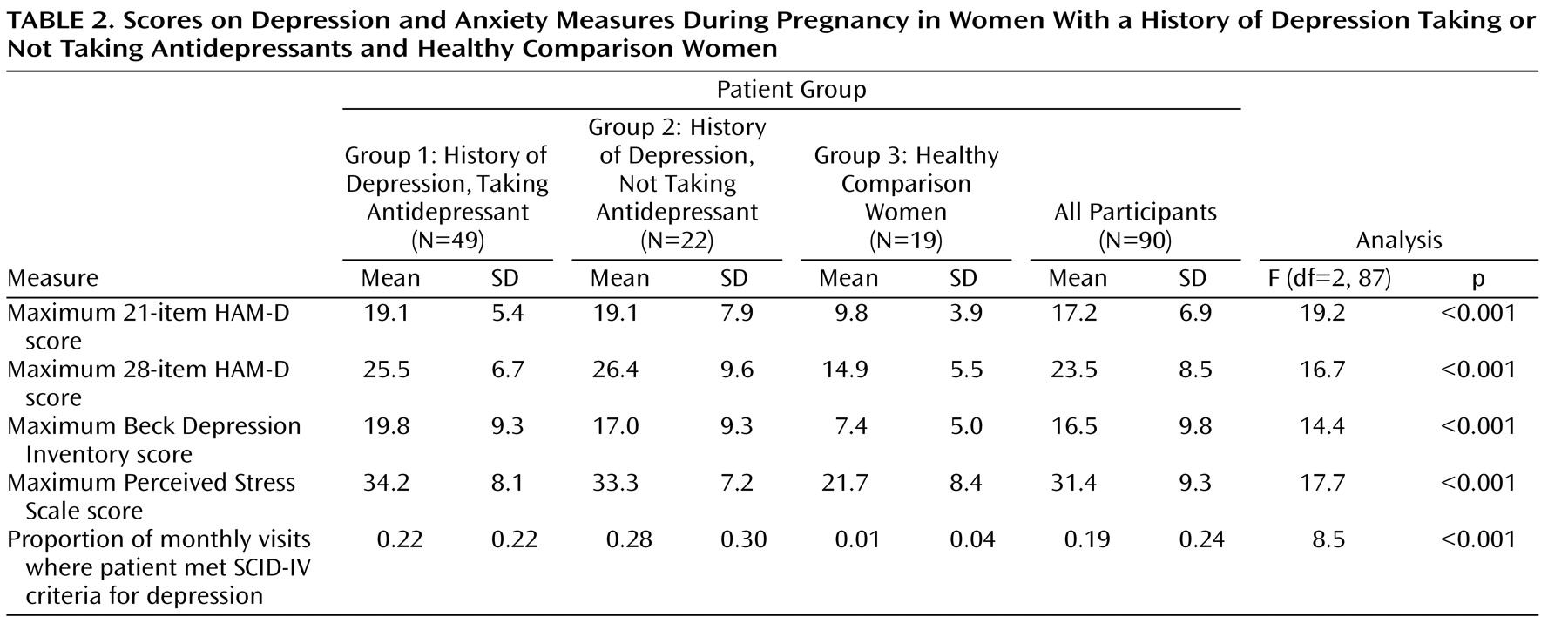
Scores on depression and anxiety measures are presented in
Table 2 . Groups 1 and 2 met SCID criteria for major depression for 22%–28% of visits on average, with a maximum mean 21-item HAM-D score of 19.1. The control group met SCID criteria for depression for 1.0% of visits on average, with a maximum mean 21-item HAM-D score of 9.8. Post hoc t tests with Bonferroni correction showed that group 3 was significantly different from groups 1 and 2 on scales of depression and anxiety (all p<0.01). Groups 1 and 2 were comparable on all scales, with no significant differences. Thus, the two groups with major depressive disorder appear to have had a comparable duration and degree of depression during pregnancy, regardless of medication status.
Gestational Age at Birth and Preterm Birth
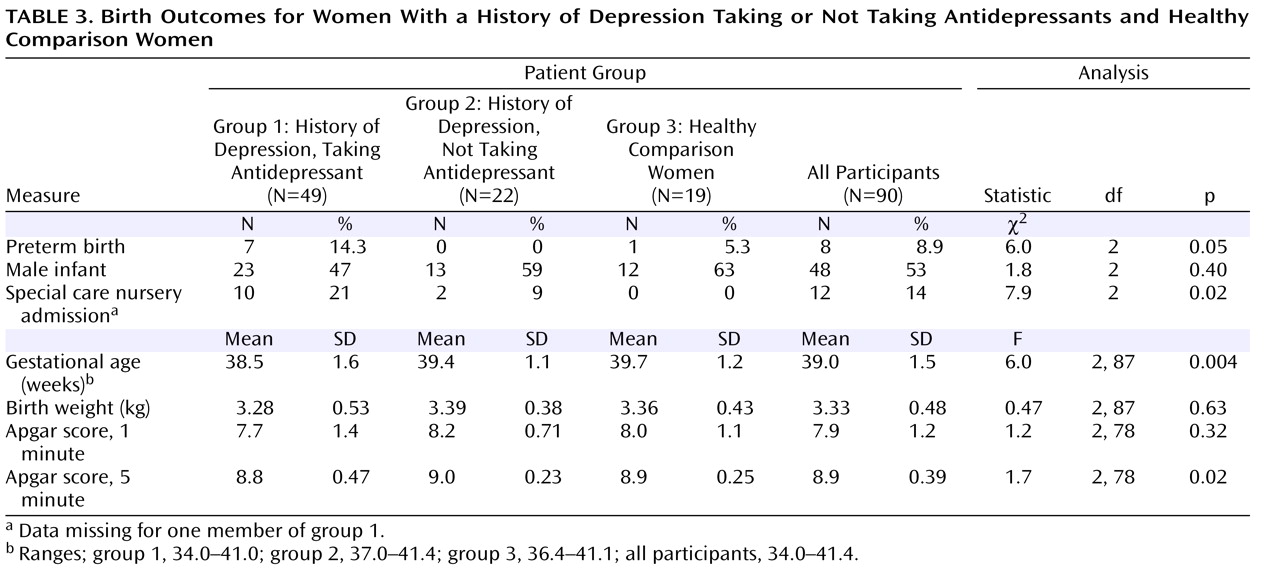
Birth outcome measures are summarized in
Table 3 . The mean gestational age at birth was 38.5, 39.4, and 39.7 weeks for groups 1, 2, and 3, respectively. The differences were significant and remained so after an ANCOVA was used to control for maternal age, number of previous pregnancies, historical and developing risk factors for preterm birth, hypertension, pregnancy-induced hypertension, and preeclampsia (F=6.0, df=2,79, p=0.004). The differences also remained significant when the analyses controlled for maternal weight gain (F=5.9, df=2,86, p=0.004). In a post hoc analysis using Bonferroni correction, infants in group 1 were found to have been born significantly earlier than infants in group 2 (t=2.46, df=69, p=0.05) and group 3 (t=3.05, df=66, p=0.01). Gestational age at birth was not significantly different, however, between groups 2 and 3 (t=0.61, df=39, n.s.). Rates of preterm birth were also significantly different between the three groups, with 14.3% for group 1, 0% for group 2, and 5.3% for group 3 (
Table 3 ). The mean gestational age at birth for the preterm births was 35.6 weeks (SD=1.1). Antidepressant exposures among the preterm births included sertraline (N=4), citalopram (N=1), fluoxetine (N=1), and nortriptyline (N=1).
Other Variables of Birth Outcome
Between-groups differences in sex of the infant, Apgar scores, infant birth weight, and multiple births (there was one case of multiple births, a set of twins in group 2) were not significant. After analyses controlled for variables that could influence birth weight (prepregnancy body mass index, weight gain in pregnancy, parity, sex of the infant, and gestational age at birth), between-groups differences remained nonsignificant (difference in birth weight between groups 1 and 3: 141 g (SD=120); difference in weight between groups 2 and 3: 111 g (SD=130). Admissions to the special care nursery were significantly different, with 21%, 9%, and 0% for groups 1, 2, and 3, respectively.
Antidepressant Dose and Birth Outcome
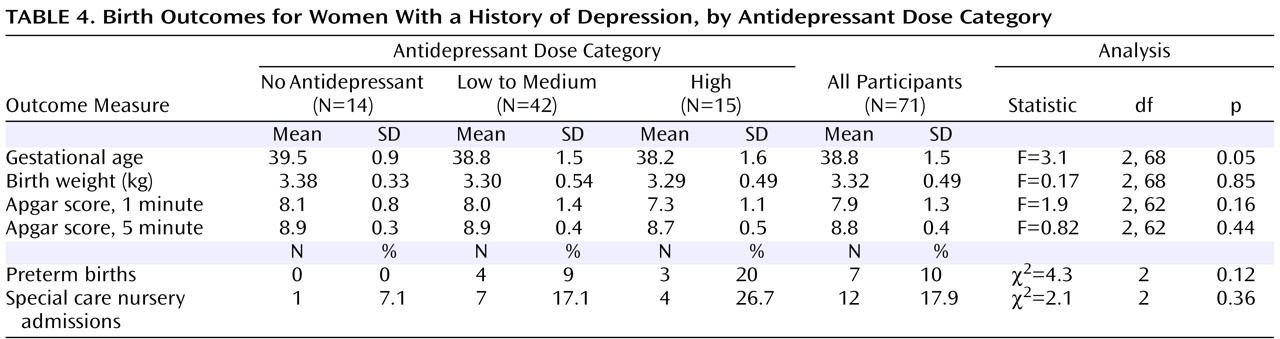
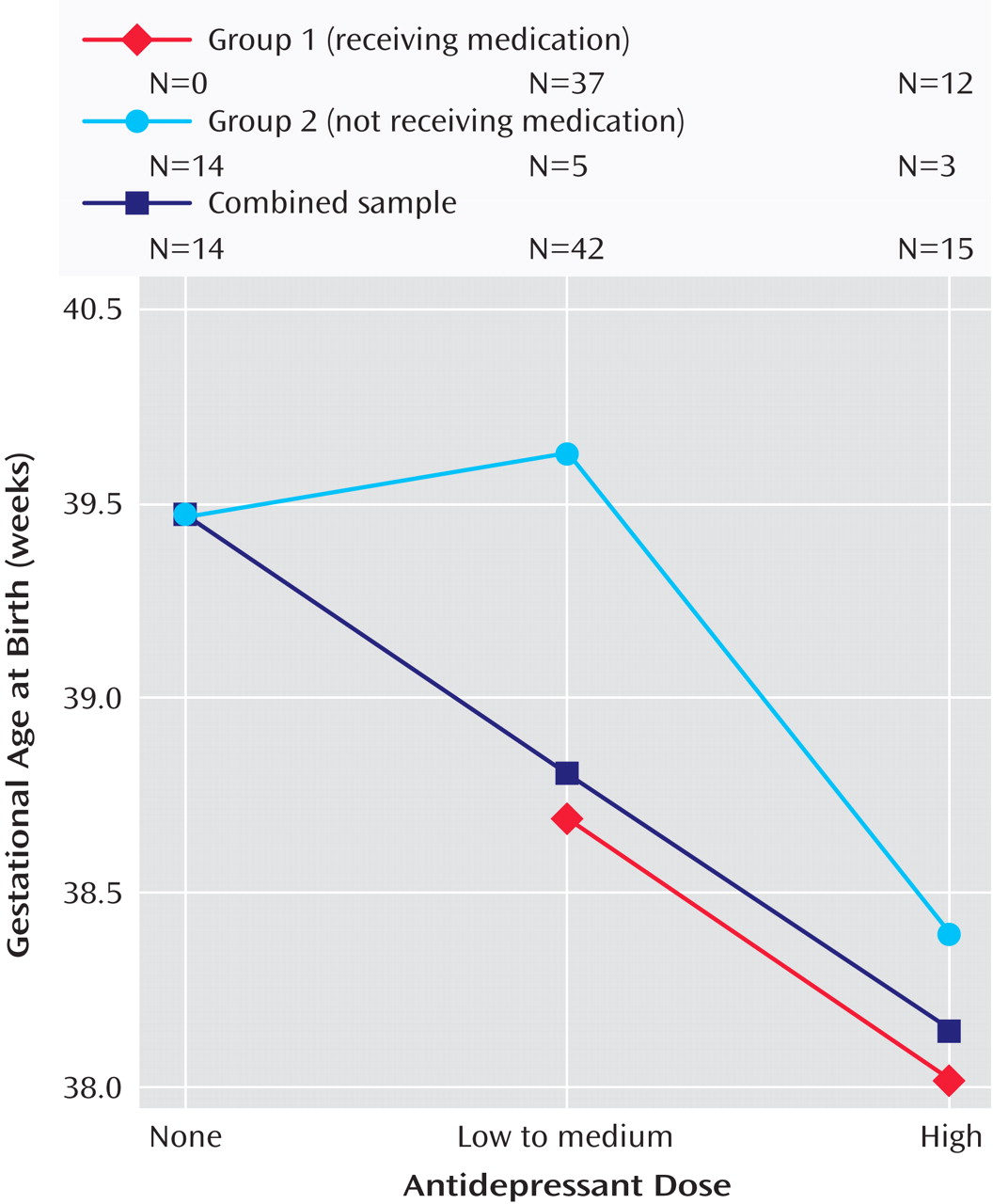
To examine the impact of antidepressant dose on gestational age at birth and on risk of preterm birth, we divided participants with depression into three groups: those with no antidepressant exposure, those with exposure to low to medium doses of antidepressant, and those with exposure to high doses of antidepressant. The dose category was determined by the highest dose the participant took, for any length of time, during pregnancy. High doses were defined as those ≥40 mg of citalopram, ≥20 mg of escitalopram, ≥40 mg of paroxetine, ≥40 mg of fluoxetine, ≥150 mg of sertraline, ≥225 mg of venlafaxine, ≥300 mg of bupropion, and ≥100 mg of nortriptyline. Doses below these levels were categorized as “low to medium.” Gestational age at birth was significantly different between groups, with 38.2 weeks for the high-dose group, 38.8 weeks for the low-to-medium-dose group, and 39.5 weeks for the no-antidepressant group (
Table 4 ). These differences remained significant after the analyses controlled for maternal age, number of prior pregnancies, and number of historical and developing preterm risk factors (F=3.3, df=2, 63, p=0.05) as well as maternal weight gain during pregnancy (F=3.1, df=2, 66, p=0.05). As shown in
Figure 1, the relationship between gestational age at birth and antidepressant dose category suggests a linear relationship, with higher medication dose associated with lower gestational age at birth (combined sample). Further examination of this linear relationship with a test of one degree of freedom demonstrated significance (F=6.3, df=1, 69, p=0.01), which remained after including in the analysis maternal age, number of prior pregnancies, and number of preterm risk factors (F=8.5, df=1, 65, p=0.005) as well as maternal weight gain during pregnancy (F=6.3, df=1, 67, p=0.017). When the analysis was restricted to group 1 participants, gestational age at birth was 38.7 weeks (SD=1.5) for the low-to-medium-dose group and 38.0 weeks (SD=1.7) for the high-dose group (p=0.12). The separate relationships between gestational age at birth and antidepressant dose category for groups 1 and 2 are also illustrated in
Figure 1 . When last dose at the end of pregnancy was used to define dose category (data not shown), the linear relationship between dose category and gestational age at birth remained significant (p=0.039).
The rate of preterm births was 20% in the high-dose group, 9% in the low-to-medium-dose group, and 0% in the no-antidepressant group. Between-groups differences were not statistically significant. Birth weight and Apgar scores were not significantly different between dose groups (
Table 4 ). Admissions to the special care nursery occurred at the rate 26.7% in the high-dose group, 17.1% in the low-to-medium-dose group, and 7.1% in the no-antidepressant group, and the difference between groups was not significant.
Depression Versus Antidepressant Effects on Gestational Age at Birth
A hierarchical linear regression model was used to determine the extent to which gestational age at birth could be predicted by antidepressant exposure and maternal depression status after controlling for maternal variables that may influence obstetrical outcome. Maternal age, number of prior pregnancies, and number of historical and developing preterm risk factors did not account for a significant amount of variability in gestational age at birth. The inclusion of depression status over the course of pregnancy—operationalized by the percentage of visits on which patients met SCID criteria for major depressive episode—did not significantly improve the prediction of gestational age at birth (R 2 difference <0.01, n.s.). The overall model, including all the above variables, did not significantly account for gestational age at birth (R 2 =0.06, n.s.). However, adding antidepressant status as an independent variable—operationalized as the percentage of visits the patient was taking an antidepressant—increased the amount of explained variance to an R 2 value of 0.19, demonstrating that antidepressant status has a significant effect on gestational age at birth (R 2 difference=0.13; F=13.0, df=1, 82, p<0.01), beyond the impact of depression and maternal risk factors.
Discussion
This study is unique in its prospective design; the inclusion of generally healthy subjects with good prenatal care and without confounding factors, such as use of other substances and medications that may adversely affect the fetus; the inclusion of a comparison group; the quantification of antidepressant use during pregnancy; and monthly prenatal mood ratings. We found that the gestational age at birth for the babies of women with depression who were treated with antidepressants during pregnancy was significantly lower than that of babies of women with depression who were not treated with antidepressants and of a comparison group of women without depression. Higher doses of antidepressant were associated with lower gestational age at birth than were lower doses.
We did not find an adverse effect of depression during pregnancy on gestational age at birth. This result was surprising to us, as we had anticipated that depression and anxiety during pregnancy would be associated with an increased risk of preterm birth. The two groups of women with depression—those who were treated with antidepressants and those who were not—had similar degrees of depression and anxiety during pregnancy. Thus, we found that antidepressant use, not mild to moderate depression, was associated with lower gestational age at birth and an increased risk of preterm birth. Our study subjects did not have severe depression, and it is possible that more severe depressive symptoms could have a negative impact on obstetrical outcome.
We found that babies of women who took antidepressants had a higher rate of admission to the special care nursery. These special care nursery admissions must be distinguished from neonatal intensive care unit admissions, which suggest more serious concerns about an infant’s health and for which data were not obtained in our study. It is possible that the significantly greater number of special care nursery admissions for babies exposed to antidepressants in our study reflects an increased vigilance by pediatricians, admitting the infants for observation rather than because of actual perinatal difficulties. It was outside the scope of this study to use mediation analysis to separate the direct effects on an increased risk of special care nursery admissions from indirect effects through decreased gestational age at birth. However, our findings are consistent with a number of earlier studies that reported a neonatal syndrome with symptoms of increased muscle tone, tremulousness, and difficulty with respiration, feeding, and sleep in infants who had third-trimester exposure to antidepressants (36). A recent large-scale study using population health data found that prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with an increased risk of neonatal respiratory distress, even after accounting for maternal illness severity (37). In a recent case-control epidemiologic study, Chambers et al. (38) found an association between SSRI use in late pregnancy and an increased risk of persistent pulmonary hypertension of the newborn. For subjects in that study who took SSRIs during the second half of pregnancy, the risk of persistent pulmonary hypertension in their infants increased from 1–2 per 1000 to 6–12 per 1000. Exposure to SSRIs before the 20th week of pregnancy was not associated with an increased risk of persistent pulmonary hypertension. Unlike the Chambers et al. study, our study was not able to delineate the effect of timing of exposure, since 90% of the women in group 1 (those who took antidepressants) were on medication in all three trimesters, and all of the women in group 1 took medication for the last two trimesters of pregnancy.
We did not find an adverse effect of prenatal antidepressant use on infant birth weight or Apgar scores. This result was also surprising to us, since infants who were exposed to antidepressants were born earlier than those who were not. The clinical implications of an increased risk of preterm birth and a lower gestational age at birth without adverse effects on birth weight or Apgar scores is unclear, but they may be important for women on antidepressants who have other risk factors for adverse obstetrical outcomes. Our study also did not find significant differences in rates of preterm birth or special care nursery admissions by antidepressant dose groups. This lack of statistical significance may have been due to limited power and should be further examined in future studies.
Our findings are similar to those of eight earlier studies that raised concern about an association between prenatal antidepressant use and lower gestational age at birth or increased risk of preterm birth
(12 –
19) . Limitations of these earlier studies included lack of information about maternal mood state during pregnancy
(12 –
14,
16 –
19), confounding factors such as cigarette or substance use
(12 –
14,
17,
18,
19), and the use of a retrospective study design
(15,
18,
19) . These limitations, we felt, made it especially important to design a study that delineates whether it is antidepressant use per se or maternal depression that has an effect on the timing of delivery. Our prospective study showed that antidepressant exposure, independent of depressed mood, adversely affects gestational age at birth. Our data also suggest that the effect may be dose-related, with higher antidepressant dose associated with lower gestational age at birth than lower antidepressant dose. These dose findings are preliminary, however, and warrant further investigation in a larger-scale study.
Our study was limited by the use of a nonrandom design, which was selected because a randomized design with regard to pharmacologic treatment of depression during pregnancy was not considered ethical. However, the three groups did not differ significantly in demographic characteristics. Although this study was also limited by the fact that many of the women who chose treatment with antidepressants continued to have depressive symptoms during pregnancy, the impact of antidepressants was separated from depression with the use of a hierarchical regression analysis. Women in the group who were treated with antidepressants were on medication for the majority of pregnancy, and the impact of only late exposure to antidepressants was not assessed. In addition, exposure to non-SSRI antidepressants was limited in our study, and thus we are not able to specify whether the effects on gestational age at birth and risk of preterm birth can be extrapolated to all antidepressants or are primarily relevant to a specific class of medications. Finally, our depressed women who were not treated with antidepressants had mild to moderate depression and were depressed for a portion of pregnancy, and thus the impact of severe depression and/or continuous depression during pregnancy was not assessed.
Despite these limitations, our results add to the growing body of literature that suggests that prenatal antidepressant use decreases gestational age at birth and increases the risk of preterm birth. This finding may especially be significant when other risk factors for preterm birth are present. The implications of our findings must be weighed against the known high risk of relapse of depression during pregnancy for women who discontinue antidepressants. Depression is a relatively common recurrent illness with significant negative consequences in pregnancy for both the mother and her baby. Depression during pregnancy increases the risk of postpartum depression, a serious psychiatric illness that causes maternal suffering and affects infant development. Thus, any decision regarding the treatment of depression during pregnancy must be made carefully, individually weighing the risks and benefits of treatment versus lack of treatment for both mother and baby.