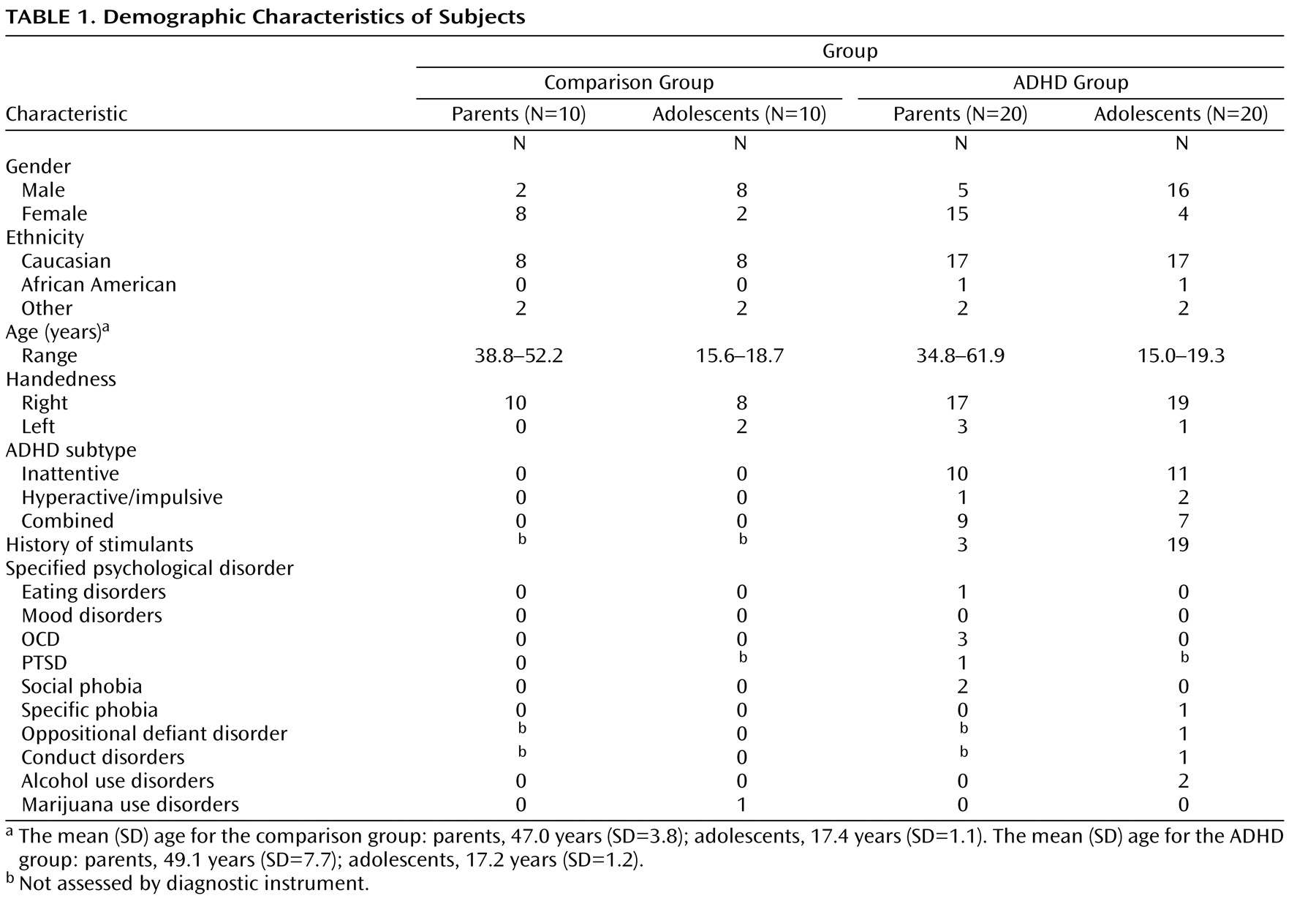
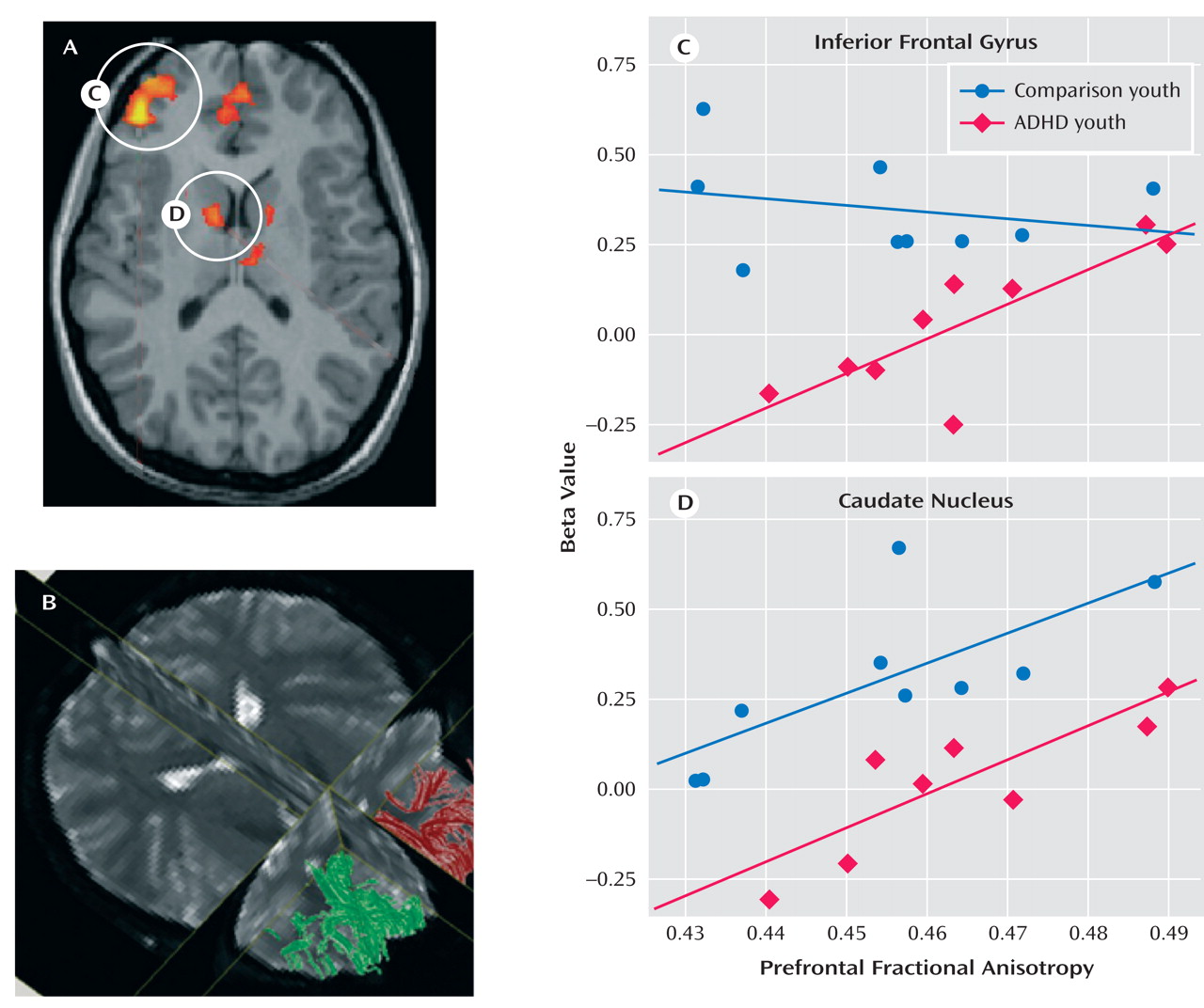
To examine the extent to which functional activity associated with go/nogo task performance was correlated with prefrontal white matter measure, functional activity in the inferior frontal gyrus and the caudate nucleus identified from the Epstein et al.
(35) study was correlated with fractional anisotropy measures in the prefrontal cortex. These functionally defined regions were selected because they were the only two regions to correlate with performance in the fMRI study of ADHD parent-child dyads. Activity in the inferior frontal gyrus correlated with fractional anisotropy in the prefrontal cortex in youths with ADHD (r=0.76, p<0.007) but not in comparison youths (r=–0.24, p<0.51) or either parent group (p<0.13 [
Figure 1 ]). Activity in the left caudate correlated with fractional anisotropy in the left prefrontal cortex in both ADHD parents (r=0.70, p<0.04) and youths (r=0.83, p<0.005) and in comparison youths (r=0.73, p<0.02 [
Figure 1 ]) but not in comparison parents (p>0.73).
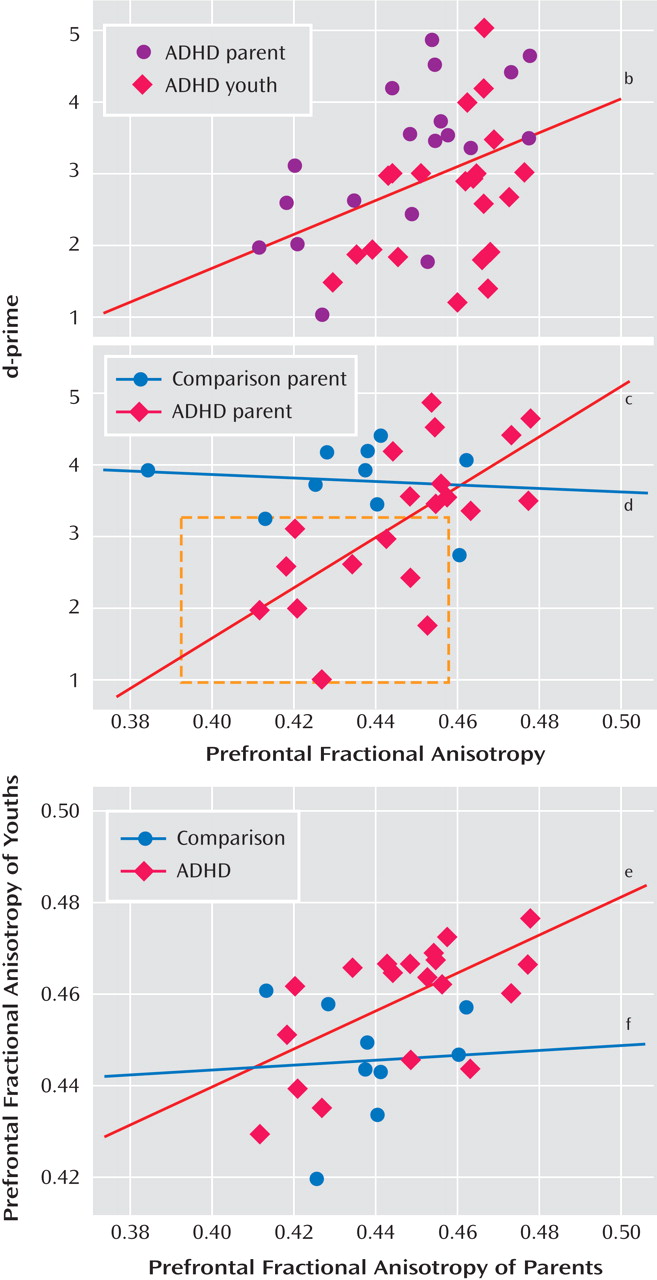
To investigate the relation between the frontostriatal white matter tracts and enhanced performance, fractional anisotropy values for individual subjects were correlated with
d- prime scores for each group separately. To determine the reliability of the diffusion tensor images-based measures of fractional anisotropy, we used three different analytic methods (voxel-based, region of interest-based, and algorithm-based fiber tracking). The findings from the algorithm-based fiber tracking method are reported, but similar associations were observed between our
d- prime measure of performance and fractional anisotropy for the region of interest-based and uncorrected voxel-based analyses. Fractional anisotropy in the right prefrontal region was correlated with
d- prime scores for the ADHD group (r=0.40 p<0.01 [
Figure 2 ]), driven largely by the correlation for the ADHD parents (r=0.65, p<0.003 [
Figure 2 ]), since youths showed only a tendency for this association (r= 0.38, p<0.09).
d- prime scores decreased in ADHD adults as prefrontal anisotropy values decreased in ADHD adults (see
Figure 2 ). Specifically, a subset of ADHD parents who performed poorly on the task had lower fractional anisotropy vaules than ADHD parents who performed as well as comparison parents. Since there was a main effect of age (F=3.79, df=1, 58, p<0.001) across all regions, partial correlations were performed controlling for the subject variable of age. The correlations above remained significant after controlling for this variable (all ADHD subjects: r=0.57, p<0.001; ADHD parents: r=0.70, p<0.001, respectively). Less significant correlations were shown between fractional anisotropy in fibers in the left prefrontal region and
d- prime scores in ADHD youth (r=0.49, p<0.03) and parents (r=0.48, p<0.04), which remained significant after controlling for age (r=0.53, p<0.03; r=51, p<0.03, respectively). There were no correlations between fractional anisotropy and d-prime for the comparison group, presumably because of less variance in behavioral performance, and there was no diagnostic group-by-region interaction (F=1.85, df=3, 171, p<0.14).
A segment of the corticospinal tract that was not expected to have any direct relation to go/nogo task performance was delineated as a comparison region
(28) . Fractional anisotropy in this region did not differentiate the ADHD and comparison groups, nor did it correlate with behavioral performance (for left and right corticospinal tracts: r=–0.23, p<0.16; r=–0.05, p<0.73, respectively). Further, there was no age-by-region interaction (F=1.44, df=3, 171, p<0.23), suggesting no significant differences between the fiber tracts of interest and these comparison tracts as a function of age for this sample.
Given the correlation between fractional anisotropy in the right frontostriatal tract and performance, we examined family resemblance in this measure for the parent-child dyads. Correlations between prefrontal fiber tracts in parents and their children showed family resemblance in the ADHD dyads (see
Figure 2 ). There was a positive association between the ADHD parent and child in fiber tracking-based right and left prefrontal fractional anisotropy (r=0.61, p<0.005; r=0.66, p<0.001, respectively), and to a lesser extent, in corticospinal tract (right and left regions: r=0.54, p<0.02; r=0.49, p<0.03, respectively). These associations were not present in the comparison parent-child dyads. To ensure that any significant correlations between prefrontal fiber-tract measures in ADHD parents and their children were not driven by chance or variability attributable to the disorder alone, 200 permutations of random pairings of the parent and child were conducted for each of these regions, and each was reanalyzed. Of these random pairings, two reached significance at the corrected alpha level of 0.005 for the left prefrontal regions, and none reached significance for the right prefrontal regions, except for the analysis using the actual parent-child pairing, suggesting a tight association between right prefrontal white matter fiber tracts in parents and their children with ADHD.