Study Selection
We selected randomized, controlled clinical trials for inclusion in this meta-analysis by performing a comprehensive search strategy. First, we conducted a computer-based PsycINFO search of available articles published between 1840 and March of 2005 using the following key terms to conduct title searches (asterisks denote that any characters/letters can follow the last character in the terms): cocaine, crack, opi*, heroin, amphetamine*, methamphetamine*, MDMA, ecstasy, methylenedioxymethamphetamine, cannabis, marijuana, psychedelic, mushroom, glue, inhalant, poly*, substance* abuse, substance* use, addict*, and dependen* singularly and in combination with the following secondary descriptors: treatment*, trial*, outcome*, therapy, random*, intervention*, medication*, psychopharm*, pharm*, buprenorphine, behavior*, counseling, cognitive, meta analys*, contingency, and voucher*. Second, we performed a computer-based MEDLINE search of articles available between 1966 and March 2005, combined with a Cochrane Central Register of Controlled Trials search for the first quarter of 2005 with the following search terms: substance abuse and randomized, or drug abuse and randomized, or drug dependence and randomized, or cocaine and randomized, or heroin and randomized, or opioid and randomized, or opiates and randomized, or methamphetamine and randomized, or amphetamine and randomized, or marijuana and randomized, or polydrug and randomized. These terms were searched as key, title, abstract, name of substance, and MeSH subject heading terms. This search was combined with a MeSH subject heading search in the same database with the following terms: amphetamine-related disorders, cocaine-related disorders, marijuana abuse, opioid-related disorders, phencyclidine abuse, substance abuse, and intravenous. We limited both the PsycINFO and the MEDLINE searches to studies conducted with human participants and published in the English language. All titles or abstracts for the citations produced were screened, and articles were collected for any citation that appeared to meet inclusion criteria.
Studies meeting all of the following inclusion criteria were included in the meta-analysis:
Investigations of the efficacy of any individual psychosocial treatments for substance abuse/dependence, with the exception of alcohol abuse/dependence and nicotine abuse/dependence
Randomized, controlled trials including a comparison group that could consist of inactive (e.g., waitlisted) or active (e.g., treatment as usual) treatments
Studies including adult (18 years and older) participants only
Studies including one or more of our posttreatment outcome measures (described below) to allow for comparable outcome data across studies
Investigations of the efficacy of nonintensive outpatient treatments, which we defined as consisting of no more than three 2-hour treatment sessions per week
Studies with medication as a backdrop condition were included only if the medication dosage did not vary between the active treatment and control conditions. Controlled trials comparing the efficacy of various treatment intensities (e.g., once per week versus twice per week individual therapy of the same therapy type) were excluded if a clear control group (e.g., one receiving inactive treatment or treatment as usual) was unavailable (seven studies). When multiple control conditions were included in these trials, we employed the “most intensive” treatment condition as the active treatment to compare to the control condition, and any other conditions of varying treatment intensity were excluded. Studies containing follow-up data beyond 1 month posttreatment but not presenting data at posttreatment were excluded (four studies). Likewise, we excluded studies employing control conditions known to be efficacious for substance abuse treatment (e.g., cognitive behavior therapy, five studies) and studies using trials of wrap-around service treatment (one study), therapeutic workplaces/work therapies (one study), hypnotherapy (one study), telecommunication networking (one study), brief motivational enhancement (two studies), supportive/expressive therapy (one study), and acupuncture (seven studies). In total, 30 studies were excluded from consideration.
Procedure
When available, data on a number of descriptive variables were collected for each study, including the following: sex (data from 91.2% of the studies available), ethnicity (76.5% available), employment status (64.7% available), marital status (61.8% available), average length of substance use (55.9% available), comorbid alcohol abuse/dependence diagnoses (20.6% available), numbers of weeks treatment was administered (97.1% available), number of treatment sessions per week (58.8%), number of participants entered per condition (100% available), treatment retention rates (47.1% available), and numerous outcome variables, as described below.
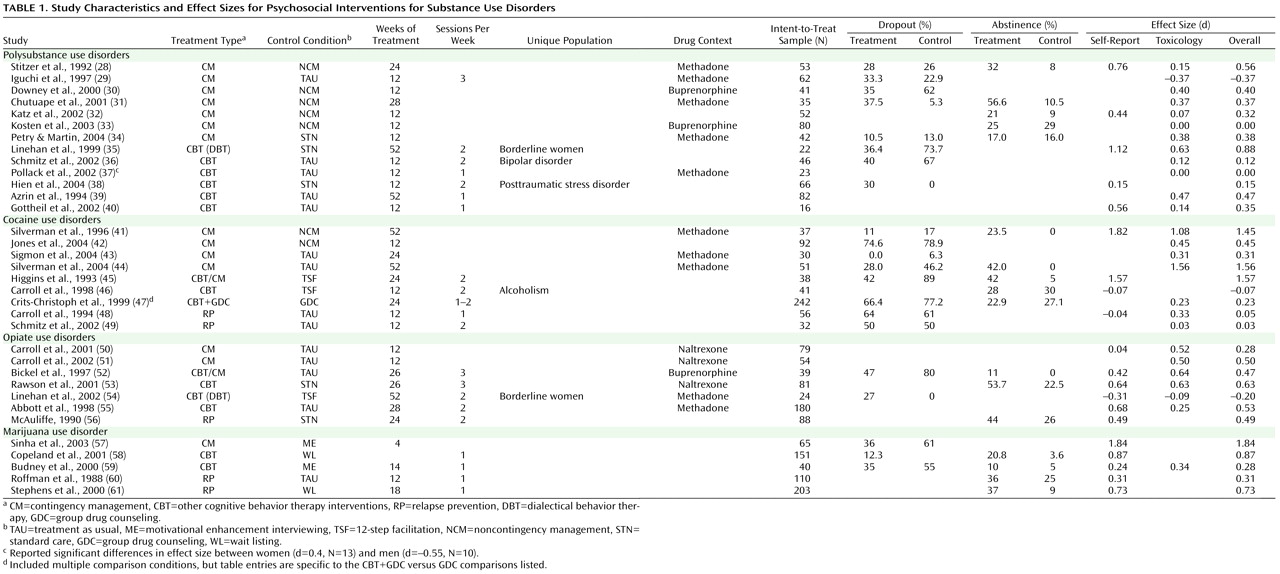
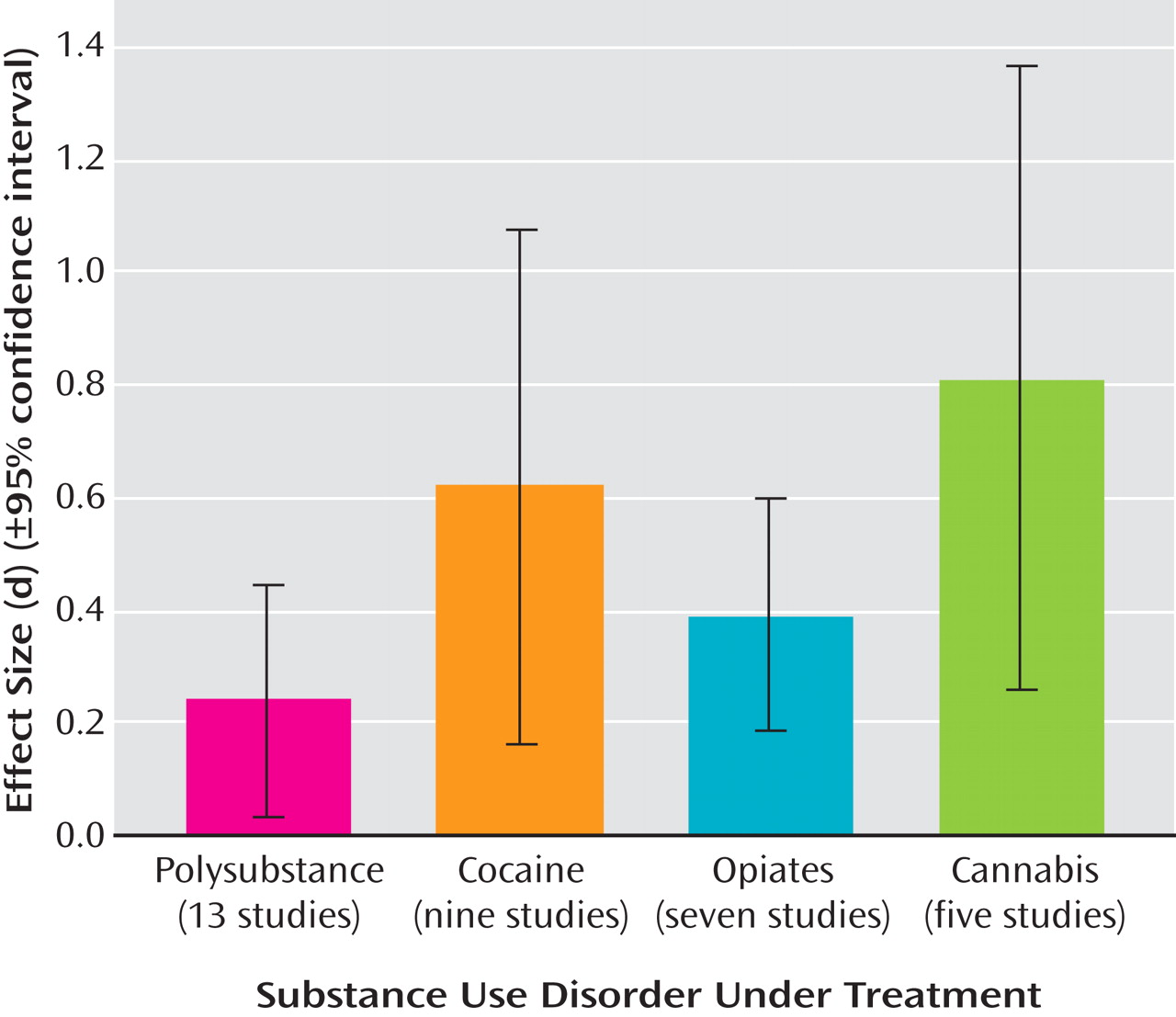
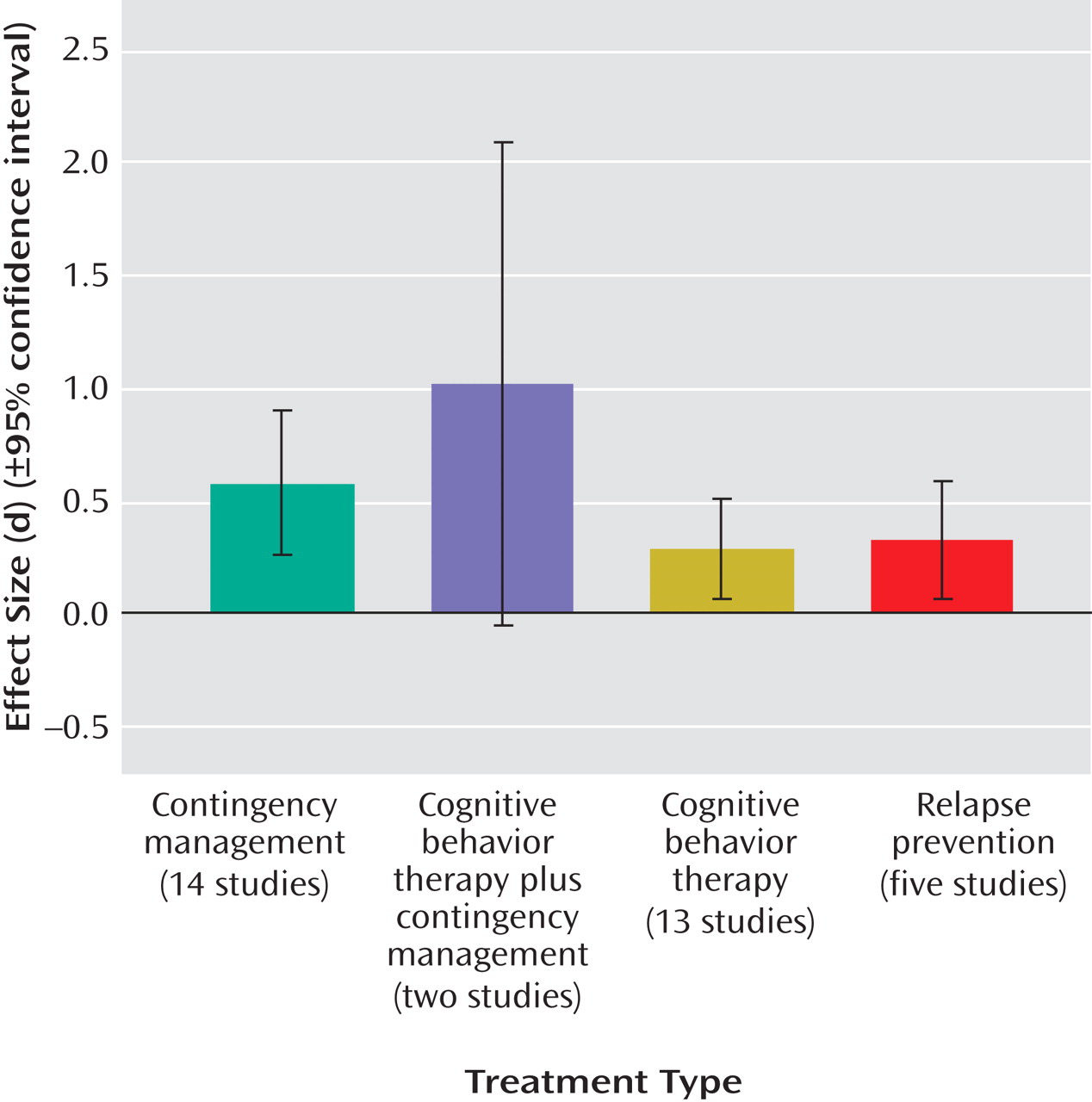
Treatments were categorized into the following treatment condition “types”: contingency management/vouchers (14 studies), general cognitive behavior therapy interventions (13 studies), relapse prevention (five studies), and cognitive behavior therapy plus contingency management combined (two studies). Descriptions of treatment conditions were used to categorize each condition into its best-fit treatment type.
Given the lack of “gold standard” outcome measures in the substance abuse treatment literature, we reviewed all controlled trials meeting our inclusion criteria, as well as all available substance abuse treatment reviews and meta-analyses, to develop a set of variables that would sufficiently allow for outcome comparison across studies. These variables included a combination of self-report data (data from 58.8% of the studies available) and measures employing toxicology screening procedures (76.5% available). Self-report outcome variables were the following:
Mean maximum number of days or weeks abstinent throughout treatment
Mean percent of days abstinent throughout treatment
Percent of sample abstinent for 3 or more weeks throughout treatment
Percent of sample demonstrating posttreatment and/or clinically significant abstinence
Posttreatment scores on the drug scale of the Addiction Severity Index
(25)Toxicology screen variables were 1) mean number of negative screens throughout treatment, 2) mean percent of negative screens throughout treatment, and 3) percent of sample demonstrating clinically significant abstinence.
With respect to the percent of the sample demonstrating posttreatment and/or clinically significant abstinence, for both self-report and toxicology screen data, studies varied widely in defining this variable. These definitions of posttreatment and/or clinically significant abstinence varied from 4 to 24 weeks of abstinence (or abstinence for the entire length of treatment), as measured by means of the participants’ self-report or toxicology screen results. The 3-week abstinence cutoff appeared to be the most widely employed measure of clinically significant change, as these data were presented by 11 (30%) of the studies included in this meta-analysis. The Addiction Severity Index (used by 16 studies)
(25) and the Time Line Follow-Back interview method (used by four studies)
(26) were the most widely used interviews for collecting self-report data across studies.
These eight outcome variables were used for the purposes of calculating treatment versus control condition effect size estimates. Effect size was calculated using Cohen’s d
(27) . Owing to inconsistency in the outcome variables employed by each study, when studies presented data on two or more outcome variables, we aggregated (averaged) across these variables to yield an aggregate mean effect size for each study. In addition, to determine the differential effect of self-report versus toxicology screen outcome data, we aggregated outcome variables within each study to yield 1) an aggregate mean overall effect size, 2) an aggregate mean self-report effect size, and 3) an aggregate mean toxicology screen effect size. Finally, as a general measure of abstinence rates, we provide the percent of the sample demonstrating posttreatment and/or clinically significant abstinence variable (these data were reported by 16 of the 37 studies), or, if this variable was not reported for a particular study, we calculated this data from the information provided (data calculated for six studies).