Despite heightened attention to the use of psychotropic medications during pregnancy and the rapidly expanding use of atypical antipsychotics for an array of mental disorders common among women of childbearing age, the reproductive safety data regarding these agents remains surprisingly limited. Similarly, there are sparse data regarding fetal exposure to these medications.
Investigations of prenatal psychotropic administration have extended to the in vivo quantitative examination of fetal drug exposure with placental passage (i.e., the ratio of umbilical cord plasma to maternal plasma concentration). Quantifying placental passage may serve to inform medication selection based on the extent of fetal drug exposure and ultimately provides insight into the extent to which neonatal complications are attributable to psychotropic exposure. It is notable that despite the introduction of antipsychotic medications over five decades ago, remarkably few data exist on the extent of fetal exposure associated with maternal use during pregnancy.
The objectives of the current study were to quantify the relative permeability of the placental barrier to various antipsychotic medications in a clinical group and to document obstetrical outcomes for women taking these agents proximate to delivery.
Method
Pregnant women treated with antipsychotics were recruited from the Emory Women’s Mental Health Program. The subjects were informed of the risks associated with fetal exposure to antipsychotic agents as well as the risks of untreated maternal mental illness. In addition, the risks and benefits of alternative treatment options were reviewed. Inclusion criteria for the current investigation included: 1) ≥18 years of age and able to provide informed consent, 2) receiving a stable daily dose of an antipsychotic for >5 elimination half-lives at delivery, 3) collection of maternal and umbilical cord plasma at delivery, and 4) laboratory confirmation of maternal compliance with the antipsychotic medication (i.e., detectable maternal plasma concentrations). Written informed consent was obtained before data collection. The institutional review board of Emory University School of Medicine approved the study.
Sample and Data Collection
Maternal and umbilical cord plasma were collected at delivery in chilled edetic acid-containing Vacutainer tubes. Because blood collected from the umbilical cord is on the fetal side of the placental circulation, the medication concentrations in umbilical cord plasma are representative of fetal exposure. Following centrifugation at 950 g for 10 minutes, the plasma was transferred to sterile polypropylene tubes, coded, and stored at –80°C until assay. The participants were followed prospectively across pregnancy at monthly intervals, at which time maternal use of prescription and nonprescription medications was documented. Obstetrical outcome data were ascertained from maternal reports and reviews of medical records.
Determination of Antipsychotic Concentrations
Quantification of the antipsychotic and metabolite concentrations was accomplished through high-performance liquid chromatography. Calibration curves were constructed for each antipsychotic drug assay with free human plasma by the addition of varying concentrations of the medications and their respective metabolites. Analysis for olanzapine and quetiapine was performed with a Waters 2690 high-performance liquid chromatography system equipped with Millennium software (both Waters Corporation, Milford, Mass.). Olanzapine concentrations were determined with previously described methods
(1,
2) . Quetiapine concentrations were determined with a modified version of the olanzapine methods with respect to the wavelength (i.e., ultraviolet wavelength was changed to 210 nm). An ESA CoulArray model 542 with CoulArrayWin software (ESA Biosciences, Inc., Chelmsford, Mass.) was used for the analysis of haloperidol and risperidone. Haloperidol concentrations were determined with a previously established method
(3) . The risperidone extraction method was based on an existing method
(4) . Separations for risperidone and its metabolite were achieved at a flow rate of 1.0 ml/minute with a Waters Novapak C18 reversed phase column (Waters Corporation, Milford, Mass.) (3.6×150 mm, 5 mm). The mobile phase consisted of 27% acetonitrile in 50 mM sodium phosphate (monobasic/Ultrapure) with a pH of 4.5 (unaltered). A two-channeled CoulChem electrochemical cell (ESA Biosciences, Inc., Chelmsford, Mass.) model 5010A was used with the cell potentials set to 500mV and 975mV. The retention times for risperidone and its metabolite, 9-OH-risperidone, were 16.8 and 11.2 minutes, respectively.
Data Analysis
An initial demographic analysis with frequency tests for categorical variables and analysis of variance (ANOVA) for continuous variables was conducted to characterize the group and identify potentially confounding differences among individual antipsychotic treatment groups. Placental passage was defined as the ratio of umbilical cord plasma (ng/ml) to maternal plasma (ng/ml) concentrations. The risperidone placental passage ratio was calculated from the sum of the parent compound and its active metabolite, 9-OH-risperidone, in umbilical cord and maternal plasma. The placental passage ratio was compared between medications through ANOVA. To evaluate the fetal capacity for metabolizing a psychotropic agent once it has crossed the placenta, a paired t test was performed comparing the concentration ratio of risperidone (i.e., the only agent in the current analysis with an active metabolite that was analyzed) to 9-OH-risperidone in umbilical cord plasma to that in maternal plasma.
The rates of obstetrical complications were calculated and compared between individual antipsychotic medications with frequency tests (i.e., Fisher’s exact test) for categorical outcome variables (i.e., preterm delivery, low birth weight, high birth weight, neonatal intensive care admission, neonatal cardiovascular complications, neonatal respiratory complications, and neonatal hypotonia) and ANOVAs for continuous outcome variables (i.e., 1-minute and 5-minute APGAR scores). Similar analyses were conducted to identify any association between potential confounding variables (i.e., covariates) and obstetrical complications. The association of obstetrical outcome with the following covariates was analyzed: participant demographic characteristics (i.e., race, marital status, principal psychiatric diagnosis) and concomitant pharmacotherapy with other classes of psychotropic agents (i.e., antidepressants, sedative-hypnotics, antiepileptic drugs). In this stage of the analysis, each covariate was stratified as a dichotomous predictor with the exception of principal diagnosis, which was organized into four strata: anxiety disorders, bipolar spectrum disorders, depressive disorders, and psychotic disorders. The statistical analysis of the covariates used 1) Fisher’s exact tests for categorical outcomes, 2) t tests for continuous outcomes in association with dichotomous covariates, and 3) ANOVAs for continuous outcomes in association with the polychotomous covariate. All statistical tests were two-tailed. When necessary, logarithmic data transformation was used to normalize the distribution of raw data before conduction of the ANOVA.
Results
Demographic and Clinical Characteristics
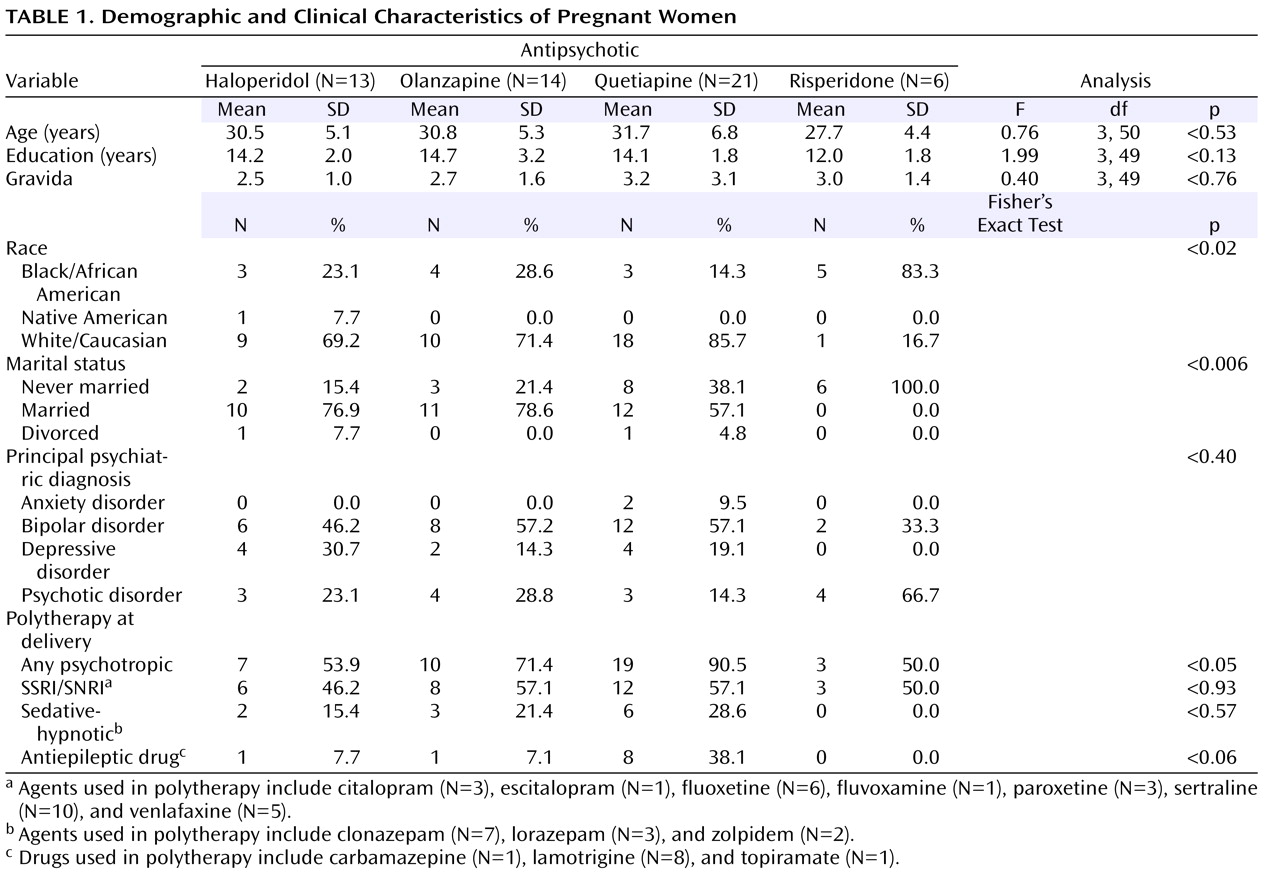
Fifty-six pregnant women treated with antipsychotics during pregnancy proximate to delivery were enrolled. One subject was excluded from analysis because of medication noncompliance as evidenced by a nondetectable maternal plasma concentration at delivery. Another was excluded because she had only been treated with the antipsychotic for 2 days before delivery. The study population was composed of the remaining 54 participants: 13 treated with haloperidol, 14 with olanzapine, 21 with quetiapine, and six with risperidone.
The demographic and clinical profiles of these 54 participants were as follows: age—mean=30.7 years, SD=5.8; 70.4% Caucasian; 61.1% married; education—mean=14.0 years, SD=2.3; and 20.4% primigravidas. Primary psychiatric diagnoses included 28 (51.9%) bipolar disorders (bipolar I disorder, N=14; bipolar II disorder, N=5; bipolar disorder not otherwise specified, N=9), 14 (25.9%) psychotic disorders (schizoaffective disorder, N=11; schizophrenia, N=3), 10 (18.5%) depressive disorders (all major depressive disorder), and two (3.7%) anxiety disorders (obsessive-compulsive disorder, N=1; posttraumatic stress disorder, N=1). Only 27.8% (N=15) of the group was receiving monotherapy proximate to delivery. Concurrent medications included antidepressants, sedative-hypnotics, and antiepileptic drugs. A stratified analysis demonstrated the risperidone group to be demographically distinct with respect to race and marital status and the quetiapine group more likely to be receiving polytherapy, particularly with antiepileptic drugs (cf.
Table 1 ).
Placental Passage Ratios
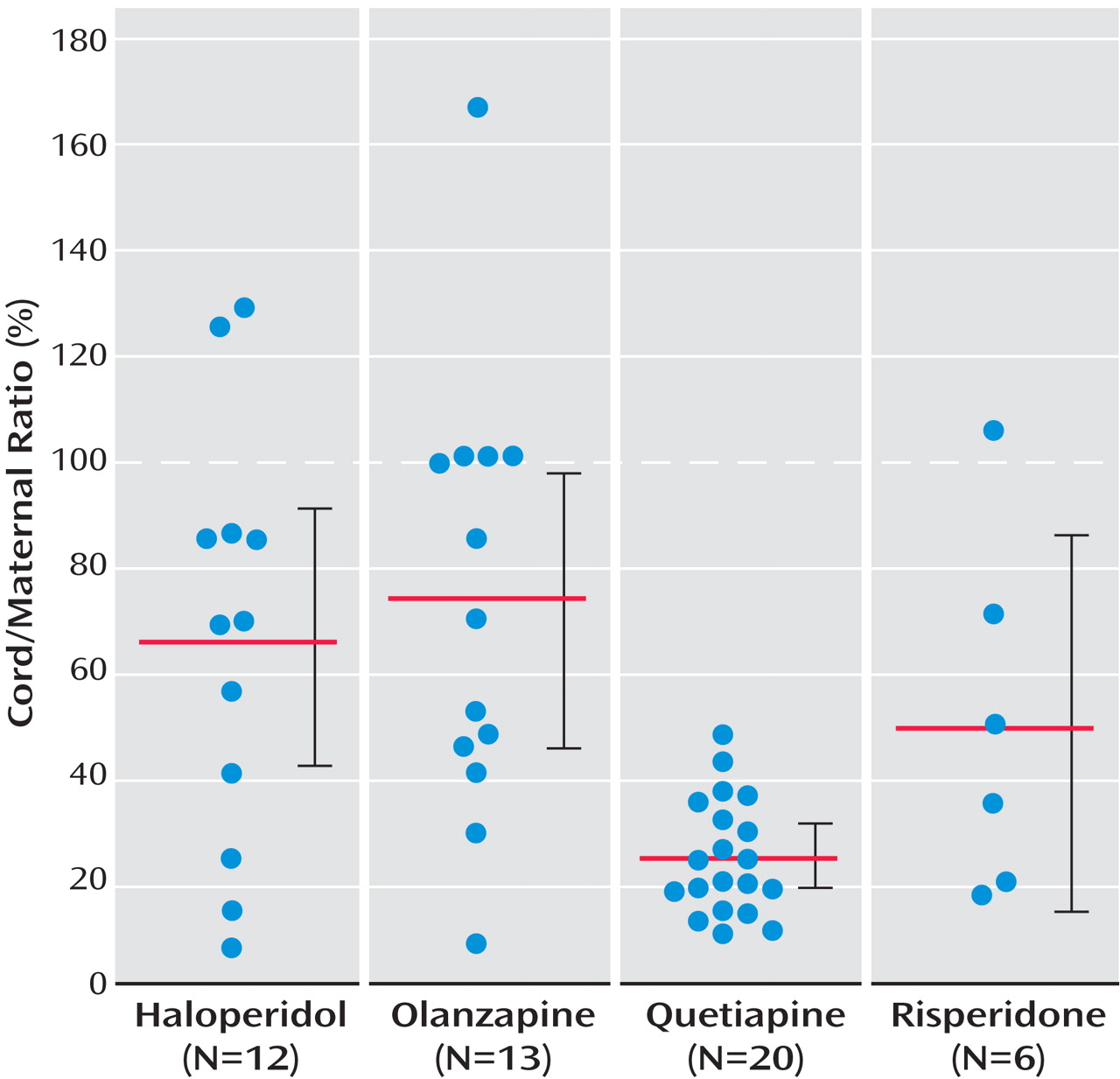
Ratios could be calculated for 50 of the 54 maternal/umbilical cord plasma sample pairs. Maternal samples were not available for two participants receiving quetiapine and one participant receiving haloperidol. The umbilical cord sample was unsuitable for assay for one neonate exposed to olanzapine. All participants had been receiving a stable dose of an antipsychotic for a minimum of 2 weeks before delivery (mean=24.2, SD=12.4, continuous weeks of exposure before delivery). Placental passage ratios, in descending order, were 72.1% for olanzapine (95% confidence interval [CI]=46.8%–97.5%), 65.5% for haloperidol (95% CI=40.3%–90.7%), 49.2% for risperidone (95% CI=13.6%–84.8%), and 24.1% for quetiapine (95% CI=18.7%–29.5%) (cf.
Figure 1 ). The placental passage ratio for quetiapine was significantly lower than that for haloperidol and olanzapine (cf.
Table 2 ). There was considerable variability in the placental passage ratios for haloperidol (range=7.0%–128.6%), olanzapine (range=7.2%–166.8%), and risperidone (range=16.8%–105.2%) but less variability for quetiapine (range=9.2%–47.0%).
Parent Compound Versus Metabolite Concentrations
Umbilical cord plasma concentrations for risperidone and its active metabolite were mean=0.36 ng/ml, SD=0.23, and mean=1.31 ng/ml, SD=0.91, respectively. Maternal plasma concentrations were mean=0.32 ng/ml, SD=0.27, and mean=3.57 ng/ml, SD=3.00, respectively. The risperidone to 9-OH-risperidone concentration ratio was mean=0.58, SD=0.66, in umbilical cord plasma and mean=0.12, SD=0.07, in maternal plasma. The results of paired t tests were not statistically significant (t=–1.69, df=5, p<0.16).
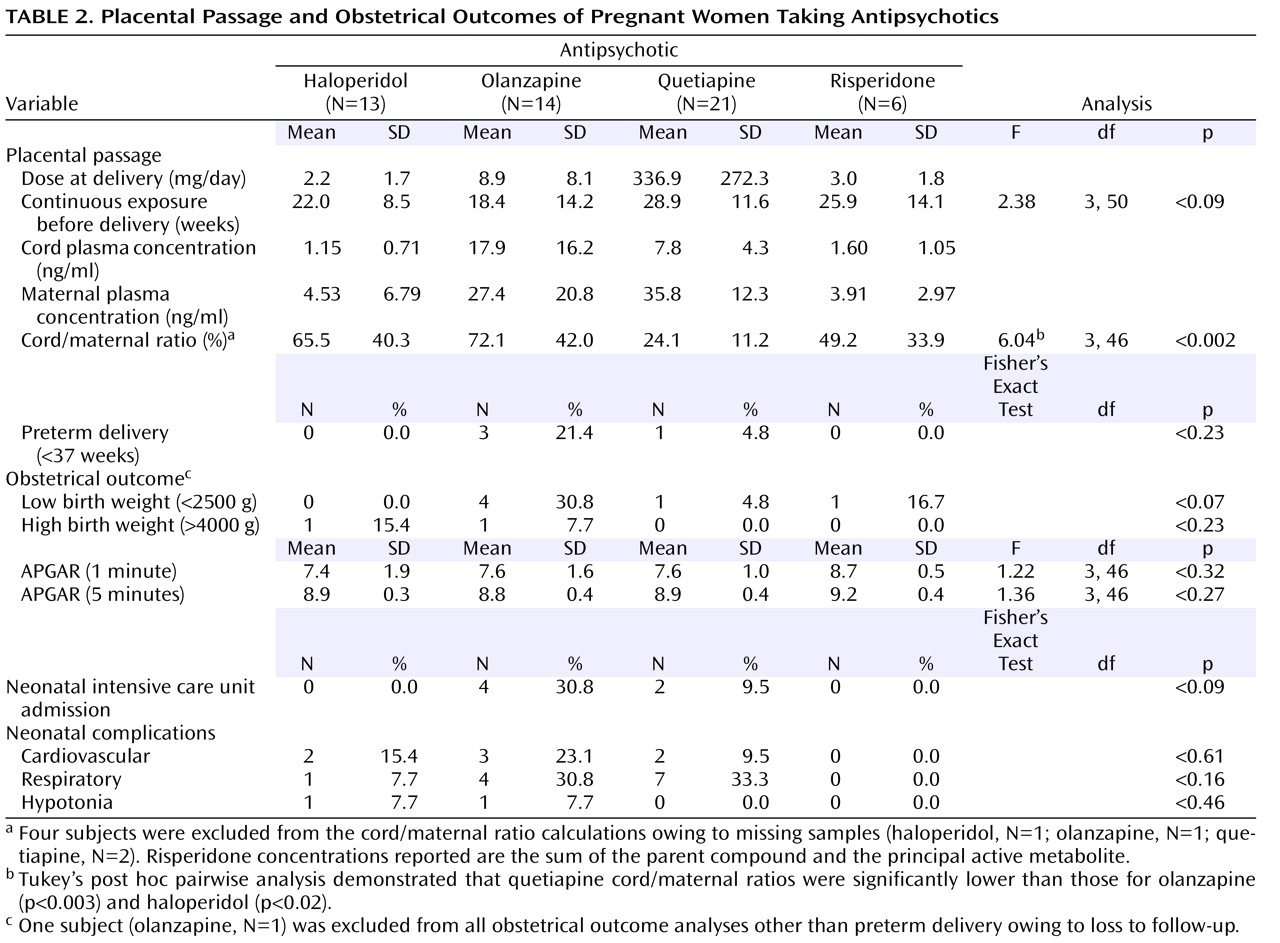
Obstetrical Outcome
Obstetrical outcome data were available for all but one of the 54 participants with laboratory-confirmed antipsychotic compliance proximate to delivery. Estimated gestational age at delivery was the only outcome variable collected for this subject, who was lost to follow-up soon after delivery. Overall, the obstetrical complications included four preterm (<37 weeks estimated gestational age) deliveries (7.4%), six low birth weight (<2500 g) neonates (11.3%), three high birth weight (>4000 g) neonates (5.7%), six neonatal intensive care unit admissions (11.3%), seven with cardiovascular complications (13.2%), 12 with respiratory complications (22.6%), and two with hypotonia (3.8%). Several adverse outcomes were higher among infants exposed to olanzapine, although the differences did not achieve statistical significance (cf.
Table 2 ). A longer duration of antipsychotic therapy (i.e., gestational weeks before delivery) did not predict adverse obstetrical outcomes (data not shown).
An analysis of potential confounding factors revealed no association between race, marital status, psychiatric diagnosis, or polypharmacotherapy and any of the obstetrical outcome variables (data not shown). Similarly, there was no association between any obstetrical outcome measure and coadministration of either a selective serotonin reuptake inhibitor/selective norepinephrine reuptake inhibitor antidepressant or an antiepileptic drug (data not shown). There was, however, a statistically significant association (p<0.007, Fisher’s exact test) between sedative-hypnotic coadministration and high birth weight. Three of 11 (27.3%) neonates exposed to a sedative-hypnotic had a high birth weight, whereas none (zero of 42) of those without sedative-hypnotic exposure had a high birth weight.
Discussion
The current study represents an initial determination of the placental passage of the newer atypical antipsychotics and provides a comparison to the typical antipsychotic that has historically been most widely prescribed during pregnancy, i.e., haloperidol. The results of this study clearly demonstrate that the placental transfer of the antipsychotics is sufficient to produce detectable umbilical cord plasma concentrations. The umbilical cord concentrations reported in the present study would likely confer considerable exposure to lipophilic fetal tissues such as the lung and the brain.
The only earlier estimate of placental transfer of atypical antipsychotics was an ex vivo placental perfusion study reporting that 5%–14% of radiolabeled olanzapine traversed the placenta over 4 hours
(5) . Consistent with our earlier studies of antidepressant placental passage
(6,
7), the in vivo placental passage ratio of 72.1% for olanzapine observed in the current study indicates that ex vivo methods may dramatically underestimate placental psychotropic medication transfer.
There were significant differences in placental transfer rates between antipsychotics. Consequently, conjecture regarding the determinants of placental passage is possible. The physicochemical properties of a drug, for example, may in part determine its placental passage. Compounds with higher molecular weights, higher plasma protein binding, and shorter elimination half-lives should arguably produce lower fetal-to-maternal concentration ratios. However, the variability in placental passage ratios observed for three of the four study medications suggests that placental passage might also be governed by patient-specific factors. Such factors might include biologically mediated maternal and fetal patterns of drug disposition and drug metabolism, pharmacokinetic interactions with other substances ingested during pregnancy, and genetic polymorphisms that have been identified for transporter proteins located in the placenta. With respect to the latter, maternal and/or fetal genotypes associated with diminished placental transporter activity might exhibit higher rates of placental passage (cf. DeVane et al.
[8] for a review). Continued in vivo sampling will enable future studies to conduct extended pharmacokinetic examinations that incorporate the potential contribution of fetal and maternal pharmacogenetic differences in placental transfer proteins and metabolic enzymes.
The nearly fivefold higher risperidone to 9-OH-risperidone concentrations in umbilical cord plasma relative to maternal plasma, although failing to achieve statistical significance in the present study, raise concerns that limited fetal metabolic capacity might result in fetal accumulation of parent compounds secondary to immature metabolic capacity. Such concerns warrant further scrutiny not only for fetal exposure but also in future investigations of neonatal medication clearance.
To our knowledge, this study was also the first to report obstetrical outcome data for those with laboratory-confirmed compliance with antipsychotic therapy proximate to delivery. Laboratory confirmation of fetal exposure is an important advance in obstetrical and neonatal outcome studies. Most existing studies of obstetrical outcome in association with maternal psychotropic treatment during pregnancy have typically relied on maternal self-reports, assuming 100% maternal compliance. The statistical tendencies toward more low birth weight babies (30.8%, p<0.06) and more neonatal intensive care unit admissions (30.8%, p<0.09) among those with olanzapine exposure also exceed population norms for low birth weight (4%–8%)
(9 –
11) and neonatal intensive care unit admission (7%–8%)
(12) . The analysis of covariate predictors, incorporating maternal demographic characteristics and coadministration of other psychotropic agent(s), identified no evidence of any likely confounding impact on these adverse outcomes. However, larger scale studies that will facilitate comprehensive multivariate analyses are ultimately needed to provide adequate control for the potentially confounding impact of maternal psychiatric illness and other clinical and demographic factors.
Excluding isolated case reports, the only existing study of acute obstetrical outcomes in association with atypical antipsychotic therapy showed a higher proportion of low birth weight babies (10%) among 105 women treated with atypical antipsychotics than among a comparator group of 105 women without psychotropic use during pregnancy (2%)
(13) . They reported that neonatal complications were not more prevalent among children in the antipsychotic exposure group. Outcomes were not reported for individual antipsychotic medications in that study.
The association of sedative-hypnotic coadministration with high birth weight contrasts an earlier study reporting lower birth weight in association with prenatal benzodiazepine administration
(14) . However, our finding is not inconsistent with a rodent study reporting that diazepam preadministration counteracted the effect of an experimental prenatal stressor that in the absence of diazepam was associated with fetal growth retardation
(15) .
The principal limitation of the present study was that the group size was not amenable to extensive multivariate analysis of the determinants of placental passage and obstetrical outcome in association with atypical antipsychotic treatment during late pregnancy. With respect to obstetrical outcome, the inability to conduct such multivariate analyses that would control for the potential confounding effects of covariate predictors limits the inferences of causality that can be drawn from the reported association between olanzapine exposure and adverse obstetrical outcomes.
These findings must ultimately be considered in the clinical context, i.e., the risks of fetal psychotropic exposure must be weighed against the growing evidence that maternal psychiatric illness poses risk to the fetus as well (cf. Newport and Stowe for a review
[16] ). Although we assessed neonatal antipsychotic concentrations in the current study for the purposes of comparative analyses, this is not recommended for routine clinical practice. First, clinical laboratories typically lack the sensitivity necessary to detect the frequently lower concentrations observed in fetal/neonatal plasma. Second, in the absence of defined therapeutic ranges or toxic levels, an infant antipsychotic plasma concentration is unlikely to be used in clinical decision making.
It should be noted that given approximately 40 years of clinical use and a reasonably extensive reproductive safety database
(16), haloperidol, from a safety standpoint, remains the preferred antipsychotic for use during pregnancy. Nevertheless, there are shortcomings to the use of haloperidol during gestation. At higher doses, coadministration of anticholinergic agents, which have been shown to carry teratogenic and other fetotoxic potential, is required
(16) . Furthermore, the therapeutic benefit of haloperidol for symptoms other than the positive symptoms of psychosis is arguably limited in comparison to atypical agents.
Indeed, atypical antipsychotics have supplanted typical antipsychotics as first-line agents for psychotic disorders, have obtained Food and Drug Administration approval in the treatment of acute mania, and are increasingly used for other psychiatric indications, including treatment-resistant depression and anxiety disorders. Not only is the use of atypical antipsychotics for an array of disorders increasingly common among women of childbearing age, but women treated with these prolactin-sparing agents, with the exception of risperidone, may be more susceptible to unplanned pregnancy than those receiving conventional antipsychotics. Consequently, data regarding the reproductive safety of these compounds bear tremendous public health implications.