While the evidence base for treatments for adolescent depression is building, little is known about the relative efficiency of such treatments. One notable exception is NIMH’s Multimodal Treatment Study of Children With ADHD, which showed that carefully implemented medication management was by far the most cost-effective intervention as compared to psychotherapy alone, medication and psychotherapy combined, or community treatment as usual
(5) . The costs of treatment are a relevant concern given the competing demands on family and health care budgets.
Earlier results from the Treatment for Adolescents With Depression Study (TADS) indicated that drug therapy (fluoxetine) both alone and in combination with cognitive-behavioral therapy (CBT) improved symptom outcomes, as measured by the Children’s Depression Rating Scale—Revised, for adolescents with depression after 12 weeks of treatment
(4,
6) . Combination therapy, however, had a greater effect size than did fluoxetine alone and had greater advantages on multiple endpoints beyond the Children’s Depression Rating Scale—Revised
(6) . CBT alone for 12 weeks did not demonstrate improved results over placebo, but it was retained for the present analysis in order to compare cost differences in all active treatment arms.
The cost-effectiveness of these treatments, however, has not yet been evaluated. The purpose of the present analysis was to determine, based on the findings from TADS, whether CBT, fluoxetine, or combination therapy is cost-effective with respect to placebo therapy in the short term and whether combination therapy is more cost-effective in the short term than therapy with fluoxetine alone.
Method
TADS was a multicenter, randomized, masked clinical trial designed to evaluate the effectiveness of CBT, fluoxetine, and their combination for the treatment of major depressive disorder in adolescents
(7) . TADS was divided into four stages, although only the acute treatment phase, stage I, is addressed here. Stage I compared four groups randomly assigned to 12 weeks of treatment with fluoxetine alone, CBT alone, combination therapy, or medical management with pill placebo. The fluoxetine and pill placebo conditions were administered double-blind while CBT and combination therapy were administered unblinded, due to the difficulties of blinding psychosocial interventions.
Participants (N=439) were adolescents ages 12 to 18 with a primary DSM-IV diagnosis of major depressive disorder and were recruited at 13 academic or community clinics between spring 2000 and summer 2003.
The present analysis focused on all TADS participants with evaluable cost and outcome data. From the initial TADS sample of 439 youths with major depressive disorder, 48 dropped out prior to completion of stage I (12 weeks). The main depression outcome measure, the Children’s Depression Rating Scale—Revised, was not available postbaseline for three individuals and 19 additional participants did not have complete data for the service utilization measurement (Child and Adolescent Services Assessment
[8] ), leaving an evaluable sample of 369 for the present analysis, which calculated incremental cost and effects based on random assignment. Of this sample, 351 completed the 12-week acute treatment stage without prematurely terminating from their randomly assigned treatment arm.
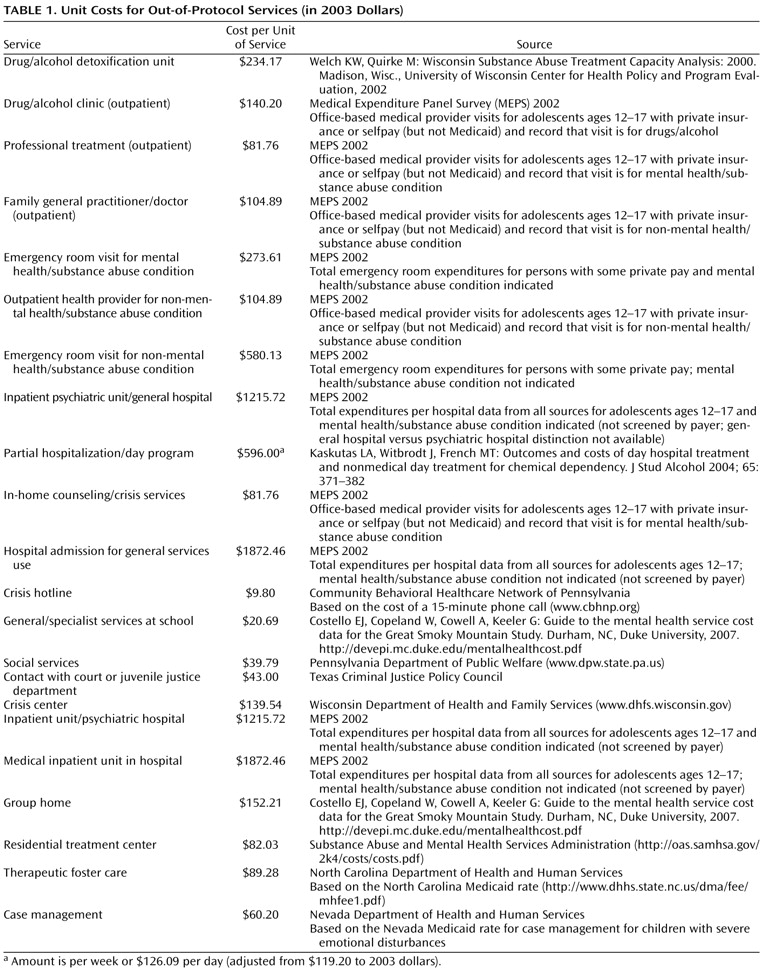
Cross-sectional incremental cost-effectiveness ratios were calculated based on initial 12-week data. Costs were generated from both in-protocol and out-of-protocol services, reported by either study staff (in-protocol) or caregivers (out-of-protocol). Costs were aggregated to the person level and reflect a societal perspective. Costs were expressed in 2003 dollars using the implicit price deflator for the Gross Domestic Product (GDP). Discounting was not used since the present study covered only 12-week costs. The cost of fluoxetine was estimated from 2003 Medicaid fee-for-service drug claim data (when generic fluoxetine was available) and was estimated to be $0.451 per 10-mg pill
(9) multiplied by the reported daily dose and days on medication; actual compliance rates were not factored in. This price was determined before Medicaid drug rebates were applied and should approximate the actual retail price. Since drug costs changed substantially during the study period (as generic fluoxetine became available in 2001) and since most participants received 20- or 40-mg/day doses, we conducted sensitivity analyses using medication costs at 25% to 50% of the initial level. Placebo pills were not given a cost. Costs per reported CBT and medication management session (placebo, fluoxetine, or combination therapy) were assigned a unit cost based on the 2003 Medicare rate ($113.09 and $59.83, respectively). Although research-related costs were not included, adverse event monitoring and adjunctive service and attrition prevention sessions
(7,
10) were also quantified using 2003 Medicare rates. Sensitivity analyses examined the influence of lower payment rates on the results. Costs of out-of-protocol services, such as inpatient hospital use and school-based counseling, were also included in the analyses. Sources for unit costs are detailed in
Table 1 . Because the focus of this analysis was on the cost of treating major depressive disorder, costs of nonantidepressant medications were not included.
In order to approximate the full costs of service use, we also quantified the time and travel costs for adult caregivers of study participants by multiplying hourly wages by the time spent traveling or waiting. We assumed that a caregiver provided transportation to study services and waited during the treatment sessions. While we do not have complete data to validate this assumption, 45%–60% of the visits with sufficient data indicate an adult in attendance, depending on the participant’s age (ages 15–18 years versus 12–14 years). Of the 14 to 15 scheduled CBT sessions in the first 12 weeks, 3 to 5 were either parent only or joint parent and adolescent sessions
(4) . Since time costs were only available for a single adult caregiver respondent, the time and travel costs for sessions with multiple adults in attendance were based on the respondent caregivers’ wages. Multiple study visits or treatments on the same day were only assigned one round-trip travel cost. No additional time costs were quantified for adolescent participants
(11) .
For individuals with incomplete dosing information in the combination therapy treatment arm (N=2), dosing was assigned based on the TADS dosing manual and the subjects’ pharmacotherapist-assigned Clinical Global Impression severity scores; in both cases we assumed the first week’s dosing was 10 mg/day, followed by 20 mg/day for the remaining 11 weeks of the 12-week period. Missing caregiver hourly wages (N=78) were imputed from the mean income of individuals matched for age, gender, level of education, and state in the U.S. Census Bureau’s 2003 Current Population Survey. Missing travel and waiting times for out-of-protocol services were determined using data from other respondents using the same service type.
The primary outcome measure was the Children’s Depression Rating Scale—Revised
(12), which was one of the two primary clinical endpoints in the primary TADS analyses
(4) . Two participants in the present analysis did not have a score at 12 weeks, so the 6-week score was carried forward. The change in scores on the Children’s Depression Rating Scale—Revised paralleled other measures of symptom change and functional improvement
(13) . To our knowledge, the Children’s Depression Rating Scale—Revised or other study instruments have not yet been translated into quality-adjusted life year (QALY) ratings, a commonly used measure which facilitates cost-effectiveness comparisons. Therefore, we translated the Children’s Depression Rating Scale—Revised scores into depression-free days
(14) and mapped the number of depression-free days into QALYs using measures previously used for adults in the literature
(14 –
16) . Depression-free days were calculated using the baseline, 6-, and 12-week values on the Children’s Depression Rating Scale—Revised. Scores were based on symptoms in the past week and were linearly interpolated between the endpoints of each period to obtain a daily score. For participants missing a score at 6 weeks (N=21), scores were linearly interpolated from baseline to 12 weeks. Sensitivity analyses excluded these individuals to examine the assumption of constant linear improvement for 12 weeks. Each daily score <29 was coded as “depression free,” consistent with the remission definition used by Kennard and colleagues
(17) . Daily scores >45 were coded as having full depression symptoms, with zero depression-free days. Daily scores ranging from 29 to 45 were coded to be proportionately depression free. Since all study participants had full depression symptoms at baseline, the theoretical maximum of 84 depression-free days was unobtainable in the present sample.
Incremental cost-effectiveness ratios express the marginal cost of one unit of improvement in the effectiveness measures. Three sets of bivariate comparisons were used to compare the cost-effectiveness of each of the active treatment arms to that of the placebo treatment arm. One thousand bootstrap replications were conducted for each paired comparison in order to generate 95% confidence interval bounds around ratio estimates. Cost-effectiveness acceptability curves
(18) were also generated, representing the proportion of bootstrap replications that were cost-effective at a range of willingness-to-pay values. We repeated the analyses for the comparison of combination therapy versus fluoxetine alone.
Differential cost-effectiveness analyses for subpopulations were conducted according to a method suggested by Hoch et al.
(19) . Moderator analyses on TADS data
(20) found that the level of family income and the severity of depression at baseline were both moderators of treatment outcomes at 12 weeks and therefore they were used to examine differential cost-effectiveness.
Results
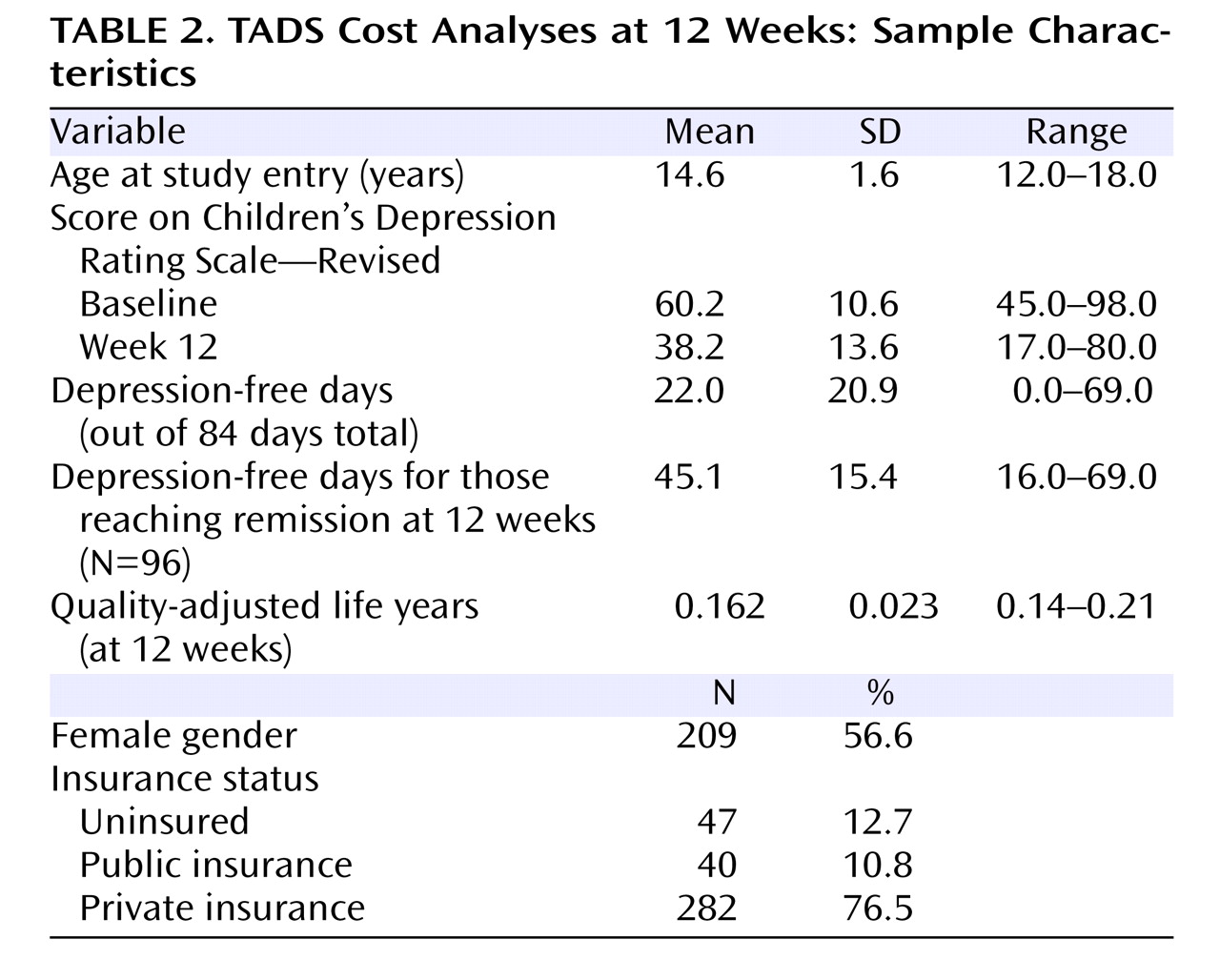
The sample (N=369) included adolescents ages 12–18 (mean=14.6 years) and was 57% female (
Table 2 ). Scores on the Children’s Depression Rating Scale—Revised decreased on average from baseline to 12 weeks. Patients experienced an average of 22 depression-free days during the 84-day period, yielding an average QALY measure of 0.16. Consistent with earlier studies
(17), 26% of the sample attained remission (defined as a Children’s Depression Rating Scale—Revised score ≤28).
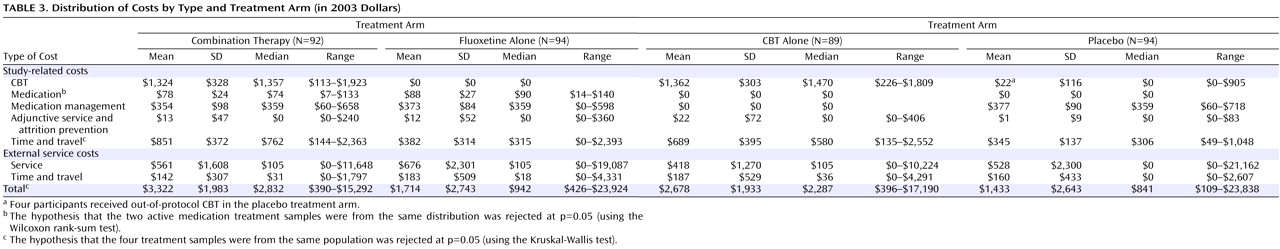
There was no difference in CBT costs between those patients receiving CBT as monotherapy and those receiving CBT in combination therapy, indicating that medication use in the combination therapy treatment arm did not alter the mean level of CBT use, as per TADS protocol
(6) (
Table 3 ). Median medication costs were significantly higher in the fluoxetine treatment arm than in the combination therapy treatment arm ($90 versus $74, p<0.01), which is consistent with the lower average dose of fluoxetine used in combination therapy. Medication management costs were quite similar (median=$359, p=0.11) across all three medication treatment arms (combination therapy, fluoxetine, and placebo). Costs of adjunctive service and attrition prevention services were minimal. Combination therapy had the highest median level of time and travel costs related to study services ($762, p<0.01), followed by CBT ($580), fluoxetine ($315), and placebo ($306). No differences were found by treatment arm for either out-of-protocol service costs (median=$0–$105, p=0.86) or out-of-protocol time and travel costs (median=$0–$36, p=0.79). The higher out-of-protocol costs for some participants stemmed largely from inpatient service use. The proportion of participants using hospital or emergency room services was similar across treatment arms (13% for fluoxetine, 9% for CBT, 18% for combination therapy, and 11% for placebo [p=0.238]). Overall, total costs for combination therapy participants were significantly larger (median=$2,832, p<0.01) than the total costs for CBT ($2,287), fluoxetine ($942), or placebo ($841).
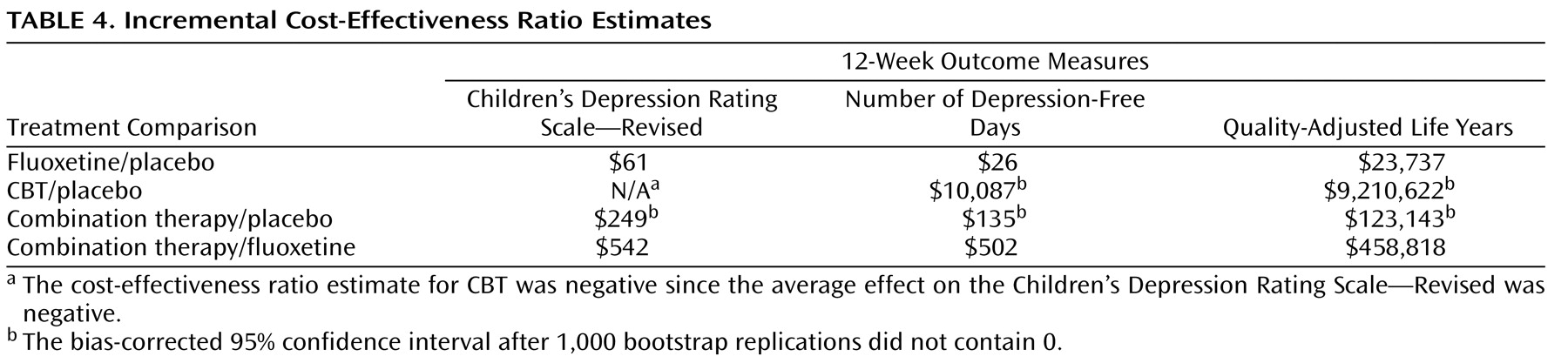
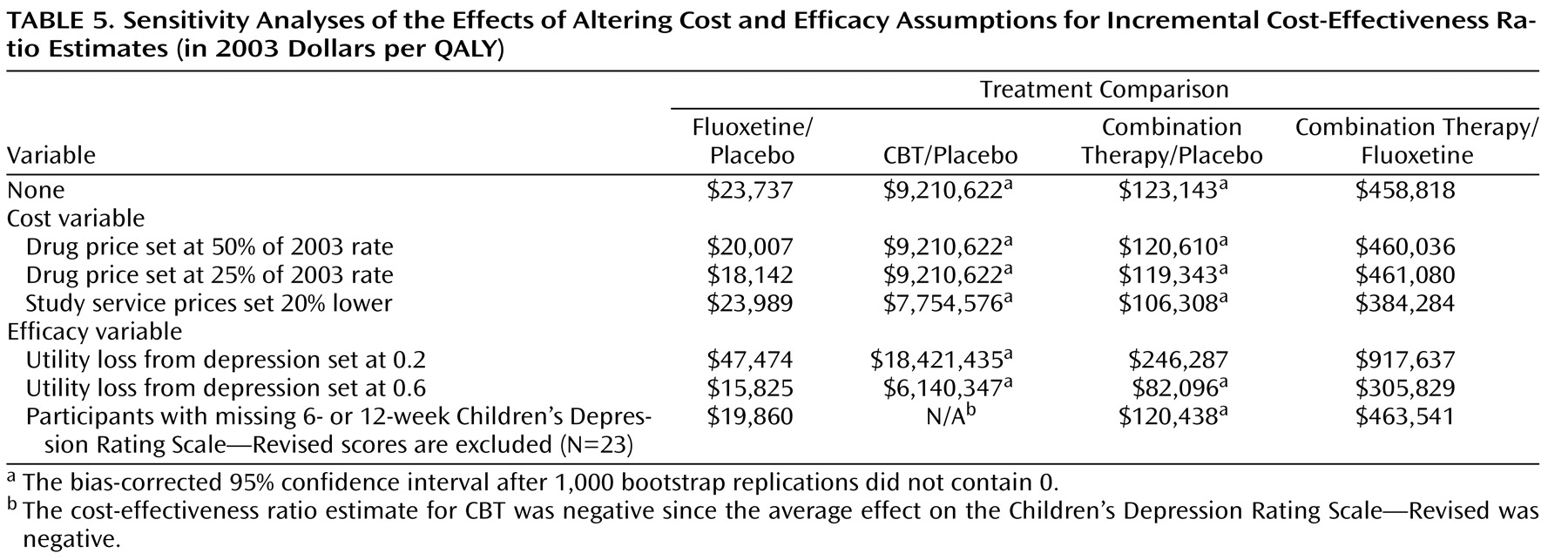
One unit of improvement on the Children’s Depression Rating Scale—Revised resulting from treatment with fluoxetine was estimated to cost almost $61 more than one unit of improvement resulting from treatment with placebo, whereas one unit of improvement resulting from treatment with combination therapy had an estimated incremental cost of $249 (
Table 4 ). In QALY terms, the results ranged from a marginal cost of almost $24,000 per QALY for treatment with fluoxetine to over $9 million per QALY for treatment with CBT alone. Both medication treatment arms (fluoxetine and combination therapy) compared favorably to placebo treatment if the threshold for cost-effectiveness was at least $125,000 per QALY
(21) . If a more conservative threshold of $100,000 per QALY was strictly applied, then only treatment with fluoxetine could be considered cost-effective.
Cost-effectiveness ratios were robust on virtually all examined permutations (
Table 5 ). The most notable difference was the greater cost-effectiveness of all active treatments if the utility loss from depression was closer to 0.6 QALYs; in this case, both fluoxetine and combination therapy treatment were clearly more cost-effective than placebo, as both fell under a threshold of $100,000/QALY.
The distribution of cost and clinical score improvements can be better observed in
Figure 1 of the data supplement that accompanies the online version of this article (available at http://ajp.psychiatryonline.org). Each point in the figure represents the incremental cost and clinical score improvement pair for a single bootstrap replication. For the comparison of fluoxetine versus placebo, incremental costs of fluoxetine were observed to be scattered around zero (77.6% positive, 23.4% negative), whereas virtually all (99.1%) of the replications showed a positive improvement on Children’s Depression Rating Scale—Revised scores. In contrast, CBT cost more on average than the placebo but showed no clear pattern of incremental improvement. Finally, estimates from the combination therapy versus placebo replications showed a clear pattern of greater clinical improvement, but at a strictly positive cost.
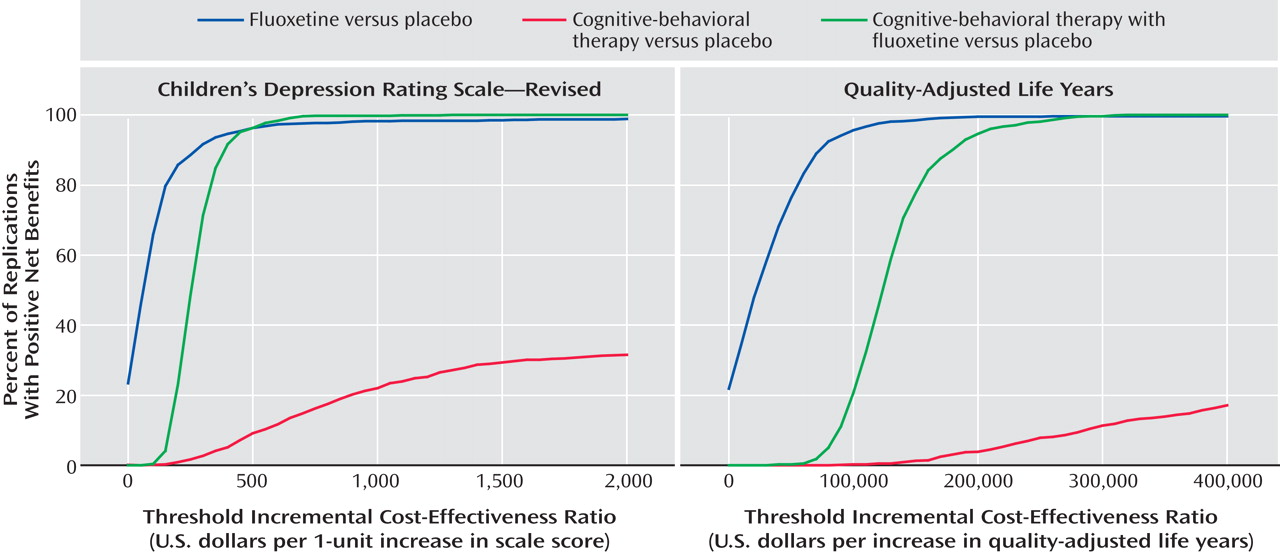
The cost-effectiveness acceptability curve provides another way to examine the results (
Figure 1 ). When clinical improvement (as measured on the Children’s Depression Rating Scale—Revised) had a low monetary value, the likelihood of a positive net benefit for any of the three active treatments was low compared with placebo. When health improvement did not carry significant societal value, an intervention was cost-effective only when it demonstrated a cost offset or net savings greater than the cost of the treatment itself. However, when clinical improvement was given a higher monetary value, both fluoxetine and combination therapy quickly became more cost-effective than placebo treatment in almost all replications. CBT treatment never reached any of the conventional levels of significance and cannot be said with reasonable certainty to be more cost-effective than placebo treatment.
Conversion to QALY ratings enabled the study results to be compared to other interventions (
Figure 1 ). If each QALY was valued at $200,000 or more, both fluoxetine and combination therapy treatment were considered cost-effective with a high degree of certainty (≥95%).
We examined the differential cost-effectiveness of the three treatment arms in comparison to placebo for subgroups defined by gender, age (16–18 years), family income (>$50,000), and baseline severity (Children’s Depression Rating Scale—Revised score >60)
(19) . We found that CBT had lower net benefits in comparison to placebo for participants with higher baseline severity. No other evidence of differential cost-effectiveness for these subpopulations was noted at the p=0.05 level.
Study participants in the fluoxetine and combination therapy treatment arms were directly compared. The bootstrapped incremental cost-effectiveness plane (data supplement Figure 2) revealed that combination therapy treatment yielded higher costs than fluoxetine, but 94% of the replications indicated greater clinical improvement. Even when society placed a high value on QALYs, a greater level of uncertainty exists than is typical when labeling an intervention as cost-effective (e.g., $400,000 per QALY; p=0.413 [data supplement Figure 3]). That is, while fluoxetine and combination therapy were both more cost-effective than placebo, combination therapy could not be considered more cost-effective than fluoxetine.
Discussion
While previous analyses from TADS examined the effectiveness of treatments for adolescent depression
(4), the present analysis adds to those results by examining how scarce resources can be better allocated among depressed youths. The unadjusted data indicate that in the short term, treatment with fluoxetine costs only slightly more than placebo (median difference of $101 in total costs over 12 weeks); fluoxetine had comparable cost profiles for time costs and out-of-protocol study services, but clearly improved outcomes. The marginal cost per QALY improvement was relatively low ($24,000/QALY). CBT was neither an effective nor cost-effective option at 12 weeks as captured by the Children’s Depression Rating Scale—Revised in TADS. Combination therapy had the highest total costs, but the greatest improvements in Children’s Depression Rating Scale—Revised scores, and should be considered cost-effective at $123,000 per QALY. The average additional gains of moving from treatment with fluoxetine only to combination therapy treatment exceeded conventional levels at over $400,000 per additional QALY. The implication of these results is that fluoxetine is more cost-effective based on short-term outcomes; combination therapy would only be recommended over fluoxetine if the additional clinical improvement is worth the markedly higher cost.
These results examined only short-term (12-week) costs and treatment effects. Longer-term studies from TADS found that while the response rate for CBT was lower than for fluoxetine or combination therapy at 12 weeks, by 36 weeks the response to CBT had caught up to that of fluoxetine (81%) and was only slightly lower than that of combination therapy (86%)
(22) . If long-term costs are proportional to those in stage I, the later CBT response could mean that CBT should become more cost-effective; whether it will pass the standard thresholds is an empirical matter. The Children’s Depression Rating Scale—Revised does not capture the full range of effects from treatment and may understate the benefit of combination therapy
(6) . Other outcomes in adolescents not examined here could also show potentially important effects of major depressive disorder treatments, including days of school missed, educational attainment, and other measures of family burden or functioning.
There are few other economic evaluations of treatments for depression in adolescents that are directly comparable to those presented here. Depression prevention with CBT in a group of high-risk adolescents for 1 year posttreatment
(16) resulted in lower costs per depression-free day and QALY. Recent reviews of economic evaluations of primary care-based depression treatment and screening in adults reported incremental costs per depression-free day ranging from $1 to $35 and incremental costs per QALY from $2,500 to $210,000 (in 2002 dollars) for studies with 6- to 24-month or greater follow up
(23,
24) . These ranges are similar to the cost-effectiveness ratios reported here, but it is not clear how comparable these studies are due to differences in populations and other aspects of study design, such as type of costs included, comparison with usual care versus with placebo, or length of follow-up period. Finally, in contrast, Haby and colleagues
(25) conducted an aggregated cost-effectiveness evaluation for children and adolescents from aggregate estimates available in the literature and found that CBT with public-sector psychologists was more cost-effective than treatment with selective serotonin reuptake inhibitors (SSRIs). However, a much narrower range of costs were considered and their model inputs included findings directly at odds with TADS results, namely that CBT was more effective than SSRI treatment.
There are a number of considerations that should be noted when placing these results in context. First, the comparison condition for the three active treatment arms was placebo, which may actually have higher costs than usual care. In particular, costs during the 3-month follow-up period for the placebo treatment arm in the present analysis (mean=$1,433) substantially exceeded the 3-month prebaseline costs of service use reported in other studies (mean=$281)
(10) . The relative effectiveness of placebo in comparison with care as usual is unknown
(26) .
A second important limitation is with respect to suicidality and other adverse effects. The potentially greater risk of suicidality accompanying antidepressant use in adolescents has received a great deal of media attention
(27) . This study did not separately incorporate either suicidal behavior or other adverse effects into the cost-effectiveness analysis, although clearly the costs of suicidal behavior in youth are tremendous
(28) . There are several reasons for this omission. First, the Children’s Depression Rating Scale—Revised may capture the extra distress associated with suicidal ideation and other adverse effects and therefore including suicidal ideation as a cost would overestimate this effect
(11) . In fact, the mean 12-week score on the Children’s Depression Rating Scale—Revised was 9.3 points higher in the six participants with reported suicide attempts. We found no difference in scores, however, between those who reported any adverse effect and those who did not. Second, the number of actual suicide attempts was fortunately too small to incorporate into statistical analysis
(4) . A simple linear probability model of suicide attempts applied to the estimation sample in the present analysis (N=369) found no difference in attempts by treatment arm (p<0.05). However, other costs of suicide attempts, such as the effect on educational attainment and labor market activities, which may not be trivial
(28), were not separately incorporated. Other limitations on study design and sample are noted elsewhere
(4,
6) . Future studies could address these concerns by including a general, rather than disease-specific, measure of quality of life that can be readily mapped to a QALY score.
Costs reported in the present analysis include broad measures of service use, not just from protocol treatments, but from settings as diverse as hospitals, medical providers, schools, and the criminal justice system, as has been recommended
(29) . While treatment decisions ideally should be based on societal costs and benefits, it is true that access to cost-effective treatments may be complicated by fiscal realities faced at the physician or patient level due to constraints on payments or provider availability. In addition, effects on parents and siblings other than time costs for caregivers during service use were not included.
The impact of major depressive disorder on quality of life is derived from the adult literature
(14,
15) . It is not clear whether the estimated loss of utility from depression used for adults adequately represents the loss for adolescents. The sensitivity analyses we conducted indicated that if depression results in greater losses in quality-adjusted life years, all treatments become more cost-effective with respect to placebo. There is some movement to either adjust life-years for different age groups
(30) or report age-specific cost-effectiveness ratios
(31) ; this analysis is consistent with the latter approach.
Treatments for adolescent depression have previously been shown to be effective. The present analysis also finds that while both fluoxetine and combination therapy are at least as cost-effective in the short term as other treatments commonly used in primary care, fluoxetine is more cost-effective than combination therapy at 12 weeks.