Among the wide range of neurocognitive deficits that characterize schizophrenia, executive dysfunction is one of the most prominent and functionally important
(1,
2) . Executive dysfunction expresses itself as impaired reasoning and problem-solving and an inability to use appropriate contextual information to generate and implement adaptive behaviors
(3) . Not surprisingly, executive dysfunction is associated with more severe and disabling forms of schizophrenia as well as poorer functional outcome
(2) . A large body of research has identified a strong association between executive impairments and structural and functional deficits in the prefrontal cortex
(4,
5) . However, we still lack a clear understanding of the etiological factors that contribute to disruption of this functionally important cognitive domain.
Increasing evidence suggests that schizophrenia has a neurodevelopmental etiology. Exposure to prenatal infections and to other obstetric complications are neurodevelopmental insults that increase vulnerability to this disorder
(6 –
9) . For example, in a birth cohort in northern California followed up for schizophrenia in adulthood, the Prenatal Determinants of Schizophrenia (PDS) sample
(10), we demonstrated that serologically documented early to midgestational exposures to influenza
(11) and elevated toxoplasma antibody
(12) were associated with schizophrenia.
To date, only a few studies have directly investigated the relationship between neurodevelopmental risk factors and cognitive deficits in schizophrenia. In those studies, obstetric complications, identified from birth records, were associated with perseverative errors on the Wisconsin Card Sorting Test, a measure of cognitive set shifting and concept formation, in both adult patients with schizophrenia
(13,
14) and healthy subjects
(15) . In another study, the combination of obstetric complications and poor performance on the Trail Making Test, part B (Trails B), discriminated patients with schizophrenia from healthy comparison subjects, including unaffected siblings of schizophrenia patients
(16) . These studies were limited, however, by the use of clinical rather than population-based samples; by the use of hospital records to identify obstetric complications, rather than systematically collected prospective data; by heterogeneous definitions of “obstetric complications”; and by incomplete data on prenatal complications. One common obstetric complication that may have accounted for some of the observed effects is prenatal infection. However, in utero infection was not documented by serologic biomarkers.
We used prospective data from a population-based birth cohort to test the hypothesis that serologically documented prenatal infections associated with schizophrenia in our previous studies would be related to impaired performance on the two measures of executive function previously reported as having associations with obstetric complications (the Wisconsin Card Sorting Test and Trails B). The sample comprised subjects from the PDS study who were recruited for the Developmental Insult and Brain Anomaly in Schizophrenia (DIBS) study, which included a comprehensive neurocognitive assessment performed on clinically stable adult patients. In secondary analyses, we investigated whether prenatal infection was associated with other executive function processes. Although we postulated that prenatal infection would affect neuropsychological performance in both schizophrenia spectrum disorders case subjects and comparison subjects, we restricted the analyses to schizophrenia spectrum disorders case subjects because of an insufficient number of exposed comparison subjects.
Method
Cohort
The cohort was derived from the PDS sample, which has been described in detail elsewhere
(10) and is summarized here. The mothers of the cohort members were enrolled in the Child Health and Development Studies (CHDS)
(17), conducted from 1959 to 1966. During that period, the CHDS recruited nearly every pregnant woman receiving obstetric care from the Kaiser Permanente Medical Care Plan (KPMCP) in Alameda County, Calif. Hence, all offspring were automatically enrolled in KPMCP. This cohort consisted of the subsample of 12,094 live births who were members of KPMCP from 1981, the beginning of case ascertainment, through 1997, the end of follow-up. Participants who remained in KPMCP and those lost to follow-up did not significantly differ from one another on several characteristics
(10,
18) .
Serologic Measures
A unique feature of the CHDS was the collection of maternal serum during pregnancy. Serum samples were stored at –20° C
(10) . The assay methods have been described elsewhere
(11,
12) and are largely reiterated here.
Influenza assay
The antigens comprised those of the prevalent influenza strains from 1959 to 1966 in northern California, including A/H2N2/Japan/57, A/H2N2/Japan/62, A/H2N2/Taiwan/64, and B/Massachusetts/66. The hemagglutination inhibition method, following Good Laboratory Practices standards as previously described
(19,
20), was used to assay the sera for antibody to influenza.
Influenza infection was defined as the first occurrence during pregnancy of an influenza antibody titer ≥20. In accord with Brown et al.
(11), influenza exposure was classified as influenza infection from day 0 after the last menstrual period until day 142 (the midpoint of pregnancy).
Toxoplasmosis laboratory assay
In accord with an established protocol
(21), toxoplasma IgG antibody titers were determined by the screen agglutination test, followed by the Sabin-Feldman dye test
(22), the reference standard
(23) . Dye test IgG titers <1:16 are considered negative. In keeping with our previous finding
(12), exposure was defined as a toxoplasma IgG antibody titer ≥1:128. The tested samples represented the last serum specimen available for each pregnancy, all of which were obtained either during the third trimester or during the perinatal period.
Because of the modest number of case subjects exposed to each of the two infections during pregnancy, we defined serologic exposure for this study as either an influenza titer ≥20 during the first half of gestation or a toxoplasma IgG titer ≥1:128. No case subjects met criteria for exposure to both infections. Two case subjects missing prenatal sera were not included in the analysis.
Ascertainment and Diagnosis of Schizophrenia Spectrum Disorder Cases in the PDS Sample
These methods have been elaborated in detail elsewhere
(10,
11) and are summarized here. The main outcome was schizophrenia and other schizophrenia spectrum disorders, defined as schizophrenia, schizoaffective disorder, delusional disorder, psychotic disorder not otherwise specified, and schizotypal personality disorder, in accordance with previous studies
(24) . Case ascertainment and screening were based on computerized record linkage between the CHDS and KPMCP identifiers from inpatient, outpatient, and pharmacy registries. Potential case subjects (patients with ICD-9 diagnostic codes from 295 to 299 and patients treated with antipsychotics) were interviewed with the Diagnostic Interview for Genetic Studies
(25) by clinicians with at least a master’s degree in a mental health field, trained to reliability. Psychiatric diagnoses were made according to DSM-IV criteria following consensus of three experienced research psychiatrists on the basis of a diagnostic interview and psychiatric/medical records. Chart review by experienced clinicians was used for potential case subjects who were not interviewed, and all diagnoses were confirmed by a research psychiatrist. These procedures yielded a total of 71 schizophrenia spectrum disorders case subjects, 44 of whom were interviewed with the Diagnostic Interview for Genetic Studies and 27 diagnosed by chart review. Prenatal sera were available for 64 of these patients. Thirty-eight patients were diagnosed with schizophrenia, 15 were diagnosed with schizoaffective disorder (thus, a total of 83% with schizophrenia or schizoaffective disorder), and 11 were diagnosed with other schizophrenia spectrum disorders.
Ascertainment of Comparison Subjects
Selection of comparison subjects began with exclusion of the schizophrenia spectrum disorders case subjects already diagnosed and of 318 patients with major psychiatric disorders other than schizophrenia spectrum disorders. Comparison subjects were matched 1:1 to the case subjects on membership in KPMCP at the time of case ascertainment, date of birth, sex, and availability of maternal sera
(10) .
Ascertainment of the DIBS Study Sample
The DIBS study is a nested case-control study. All PDS case subjects and comparison subjects matched 1:1 for neuropsychological assessments who met eligibility criteria were targeted. Exclusion criteria for both case and comparison subjects were diabetes, hypertension, history of heart disease, HIV/AIDS, cancer, autoimmune disorders, cerebrovascular disease, brain tumor, multiple sclerosis, any neurodegenerative disorder, mental retardation, epilepsy/seizures, meningitis/encephalitis, Tourette’s syndrome, autism or other pervasive developmental disorder, Parkinson’s disease, tardive dyskinesia, and history of head trauma with loss of consciousness. Case subjects who were currently hospitalized or deemed to be too severely psychotic for neurocognitive testing based on family report were also excluded. An additional exclusion criterion for comparison subjects was history or current treatment of a DSM-IV axis I psychiatric disorder, except for adjustment disorder. These case and comparison subjects were recruited in tandem with an additional protocol, which excluded individuals on the basis of obesity (defined as body weight >300 lb), and thus this criterion was applied to the protocol of the present study (only two subjects, one case subject and one comparison subject were excluded by this criterion). Selection of study subjects was not determined by maternal antibody titer.
Participants were recruited using updated information from the PDS study.
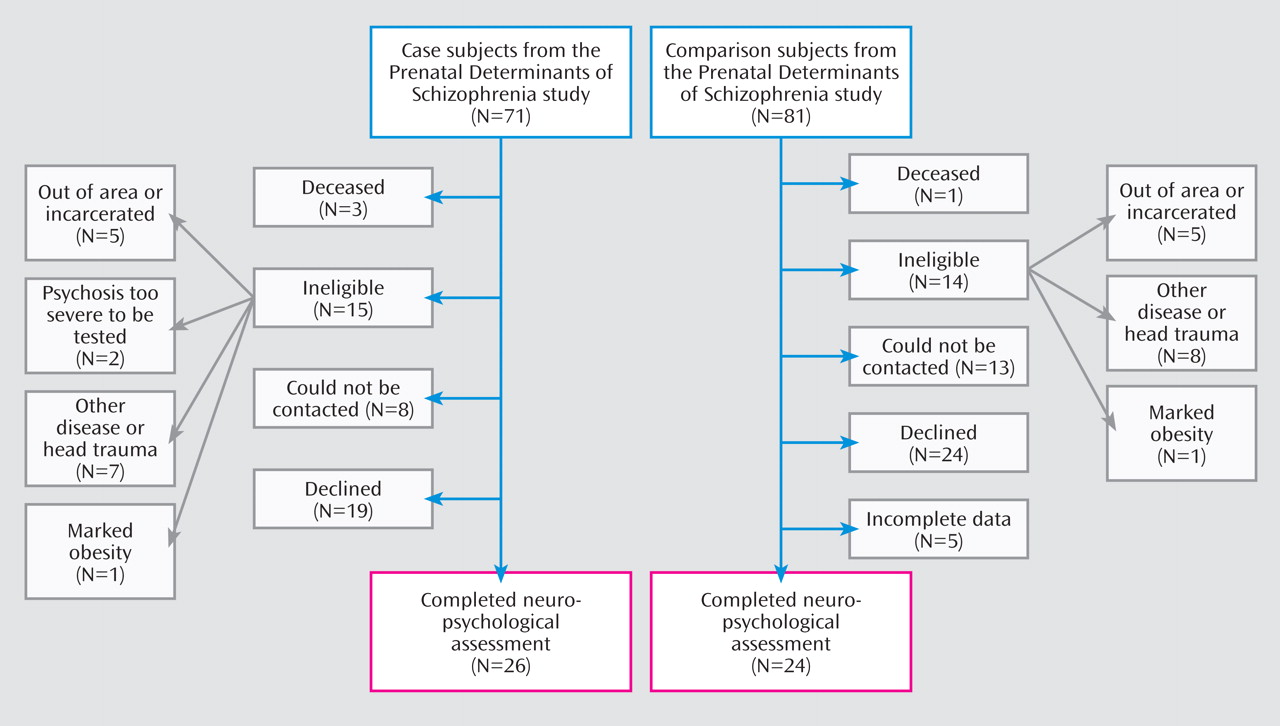
Figure 1 depicts the recruitment and selection of case and comparison subjects in the neuropsychological component of the DIBS study. Twenty-six case subjects with schizophrenia and other schizophrenia spectrum disorders (13 with schizophrenia, seven with schizoaffective disorder, and six with other schizophrenia spectrum disorders) and 24 comparison subjects were enrolled and completed the neuropsychological assessments detailed in the next section.
All participants provided written informed consent. The study protocol was approved by the institutional review boards of the New York State Psychiatric Institute; the Kaiser Foundation Research Institute; the University of California, San Francisco; and the San Francisco Department of Veterans Affairs Medical Center.
Neuropsychological Assessments
Graduate students (master’s level or higher) in a mental health-related field were trained by W.S.K. and J.H.P. in the administration and scoring of the neuropsychological tests. Detailed manuals were used to maximize interrater reliability for both test administration and scoring. The neuropsychological battery
(26,
27) included the Wisconsin Card Sorting Test; Trail Making Test, Parts A and B (Trails A, Trails B); verbal fluency (letter, category); Ruff Figural Fluency Test (figural fluency); WAIS-III digit span, forward total (digits forward) and backward total (digits backward); Wechsler Memory Scale (WMS-III), letter-number sequencing, auditory n-back (2-back, 0-back); and the reading subtest of the Wide Range Achievement Test 3 (WRAT-3). Selection of the Wisconsin Card Sorting Test and Trails tests was based on previous studies of relationships between obstetric complications and performance on these measures
(13 –
15), and selection of the remaining instruments was based on their capacity to test other aspects of executive function and working memory performance relevant to schizophrenia. The WRAT-3 reading subtest was included as an estimate of overall premorbid cognitive ability
(27,
28) .
Analyses
Two primary sets of statistical comparisons were made: case subjects versus comparison subjects, and case subjects exposed to prenatal infection versus those unexposed. The second of these analyses was restricted to the case subjects because there were too few exposed comparison subjects to permit a meaningful analysis of the data.
Before these analyses were conducted, demographic variables were dichotomized and compared by chi-square tests.
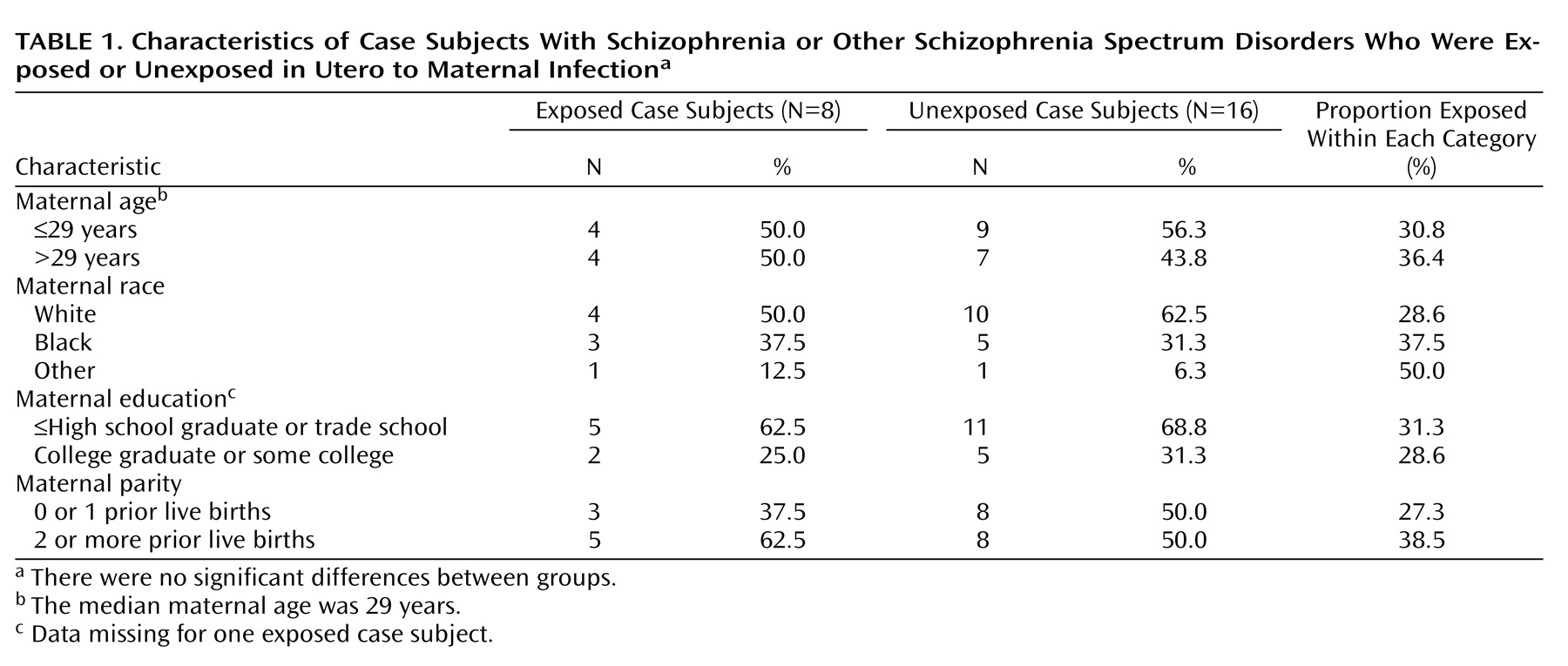
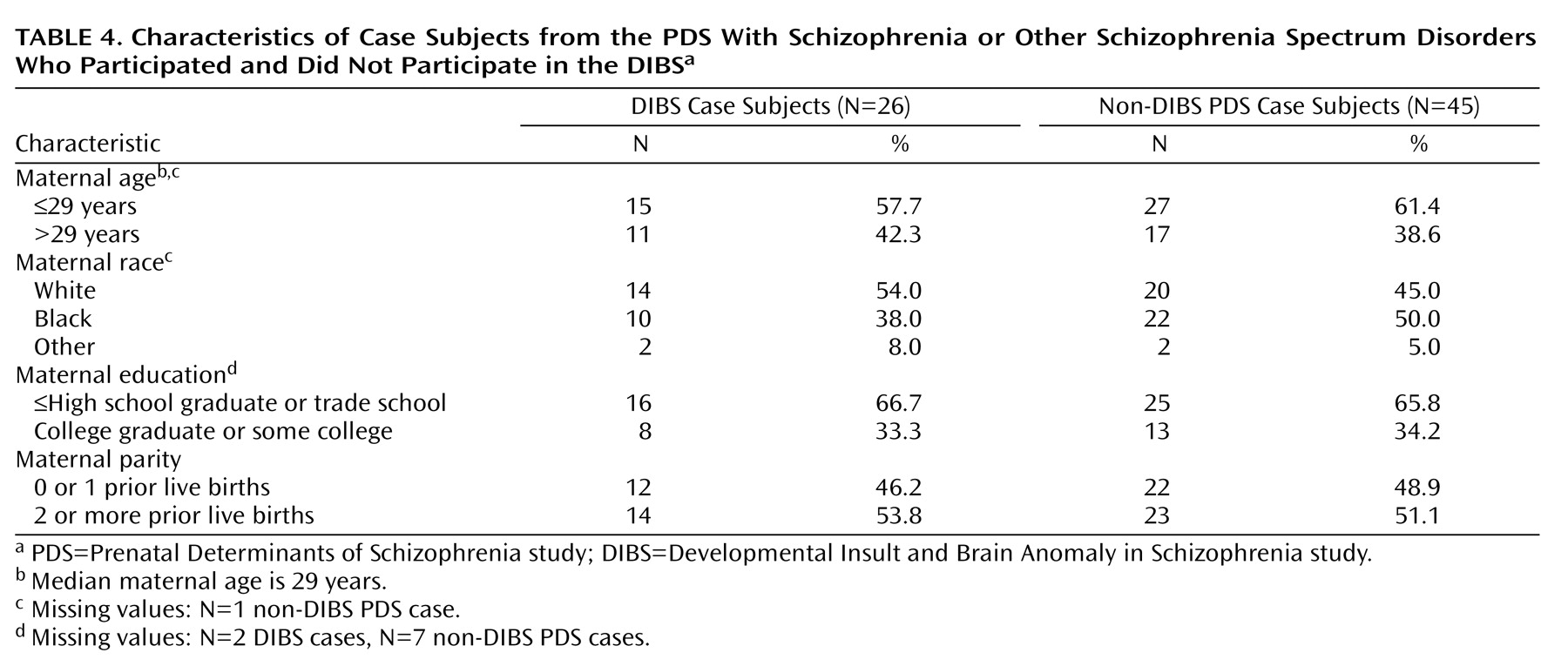
Table 1 summarizes the basic characteristics of case subjects exposed and unexposed to serologically documented infection. There were no significant differences between groups with respect to maternal age, race, education, and parity.
We observed unequal variances for certain response variables in the comparisons both of case subjects and comparison subjects and of case subjects exposed and unexposed to prenatal infection (Tables
2 and
3 ). Nonparametric methods were considered, but the sample size was insufficient to use these methods without sacrificing statistical power. While a weighted least-squares approach might address unequal variances, specification of these weights is difficult in practice. Hence we adopted a new method, generalized linear models
(29) . The generalized linear model is a flexible parametric class of models suitable for small data sets in which the conditional distribution of the response given the predictors is chosen from an exponential family (for example, Gaussian, binomial, Poisson, and gamma) and can exploit specific structure in the response variables of the study. The variance structure is determined by the particular generalized linear model, with only the Gaussian model having constant variance. For total errors on the Wisconsin Card Sorting Test as the response, a binomial regression model with 47 trials provides a reasonable approximation because the test itself is based on 47 questions, each with a binary outcome. Similarly, binomial regression is applicable for the analysis of digit span and letter-number sequencing (the number of trials are listed in Tables
2 and
3 ). For the Trail Making Test, gamma regression is suitable, as the response is measured in time in seconds. For the verbal fluency test, Poisson regression is appropriate, because there is no theoretical limit to the number of correct responses. Finally, for the auditory n-back, in which the response is a standardized continuous measurement, a Gaussian model was used. All analyses were also adjusted for WRAT-3 reading score in an effort to equate individuals on premorbid cognitive ability. In terms of the effect of the exposure on WRAT-3 reading score, there was no significant difference between groups (exposed group: mean=98.1 [SD=11.4]; unexposed group: mean=90.6 [SD=13.4]), but the mean difference of 7.5 points suggests that this could represent a meaningful difference. Moreover, this difference could obscure differences in executive function between groups since the exposed group displayed higher WRAT-3 scores, yet exposed case subjects would be expected to have poorer executive function than the unexposed case subjects. Therefore, cognitive measures were compared after controlling for WRAT-3 reading score.
Statistical significance was based on a p threshold of 0.05, and all tests were two-tailed. Effect sizes can be assessed by inspecting the confidence intervals for the regression parameter corresponding to exposure (see Tables
2 and
3 ).
Results
Comparison of DIBS Study Case Subjects and Non-DIBS Study PDS Case Subjects
Table 4 presents comparisons between PDS case subjects who participated and those who did not participate in the DIBS study. There were no significant differences between the groups on maternal age, race, education, and parity. Although not the focus of this article, we also compared these demographic variables between PDS comparison subjects who did and did not participate in the DIBS study. There were no significant differences on any of these demographic variables.
Comparison of Executive Function Measures Between Case Subjects and Matched Comparison Subjects
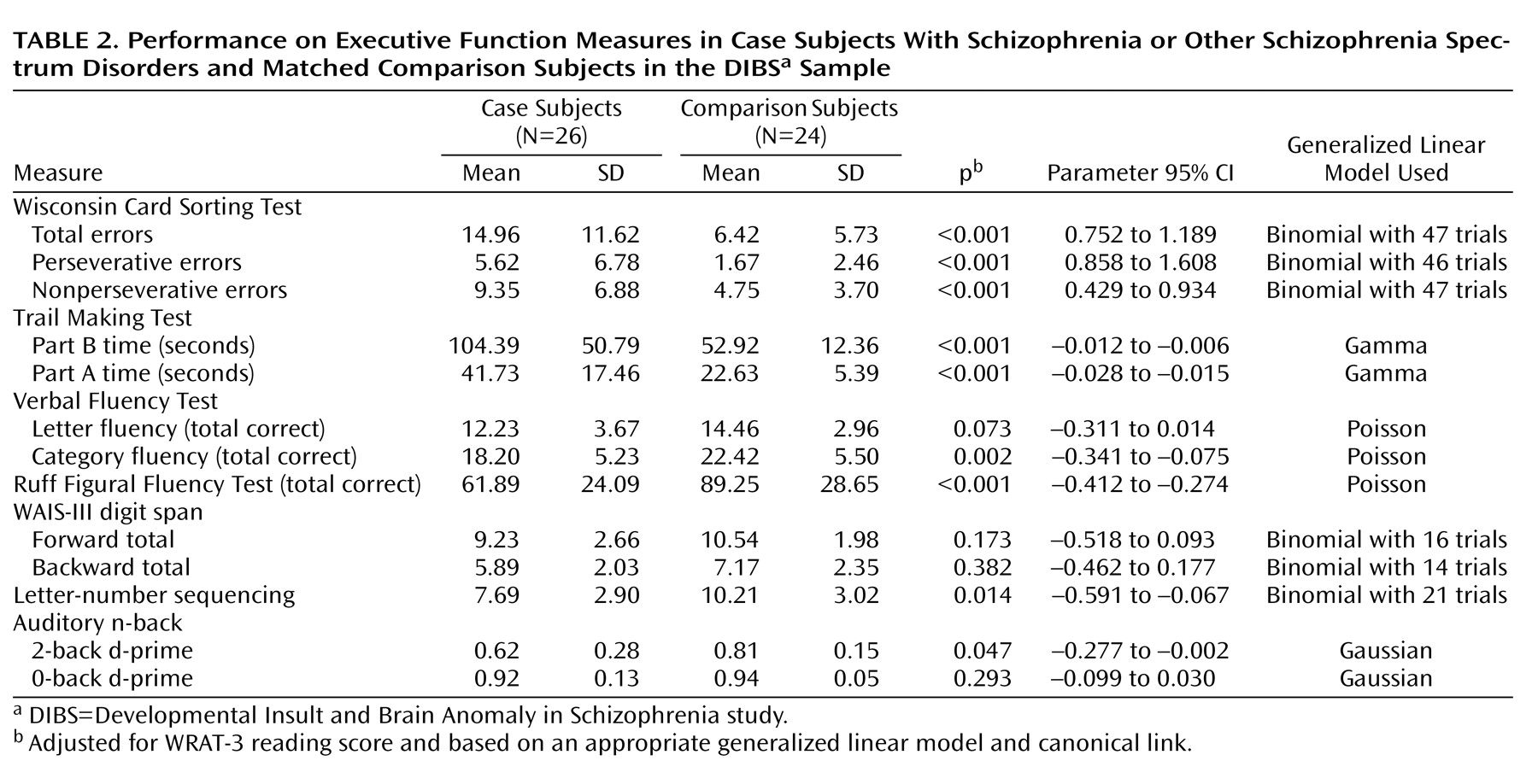
We first compared performance on measures of executive function and working memory between case subjects and matched comparison subjects in order to validate our findings against those documented in the literature. As expected, case subjects performed significantly worse than comparison subjects (see
Table 2 ). The differences reached statistical significance for nearly all tests.
Comparison of Wisconsin Card Sorting Test and Trails B Measures Between Case Subjects Exposed and Unexposed to Prenatal Infection (Primary Analyses)
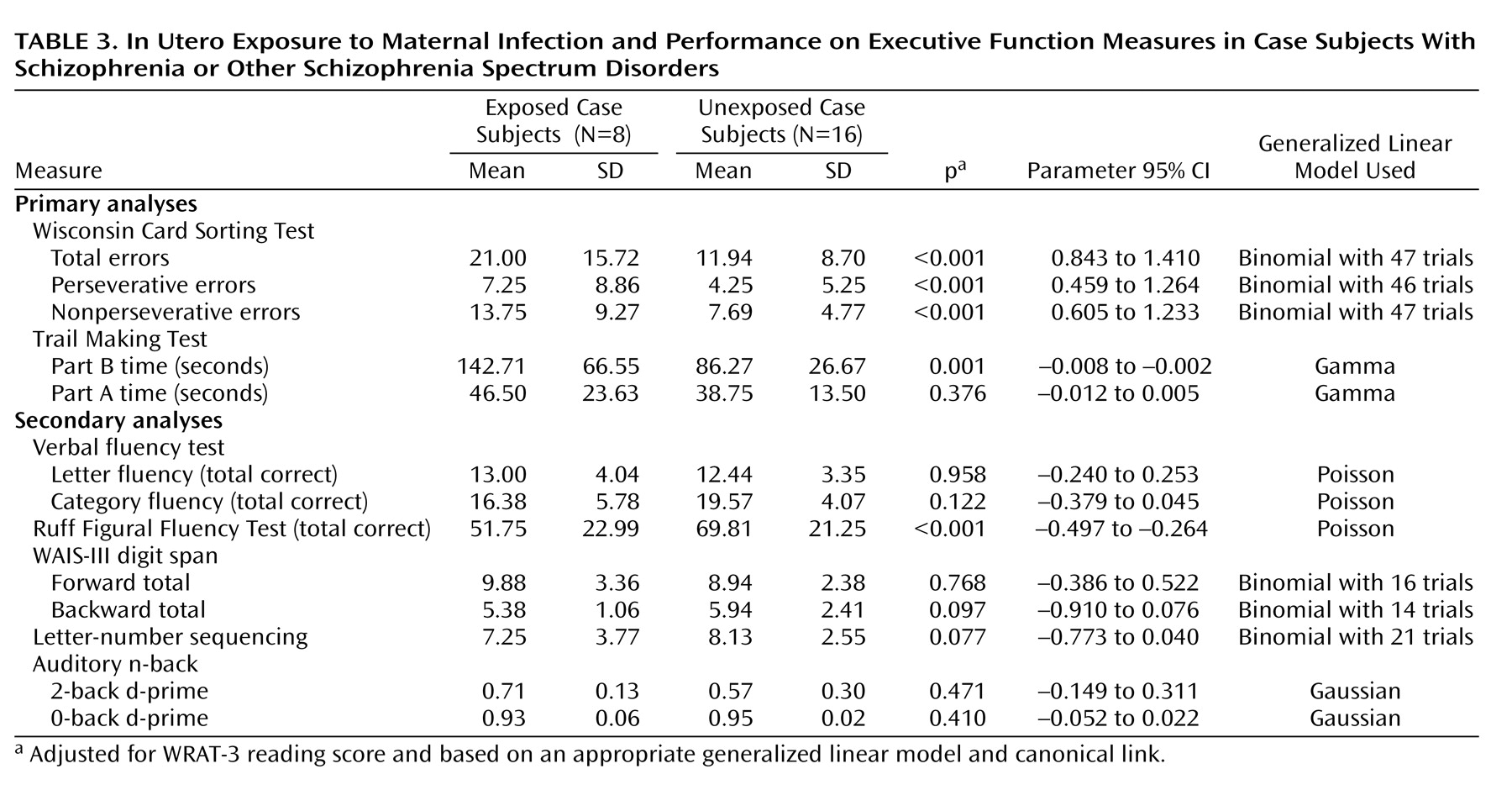
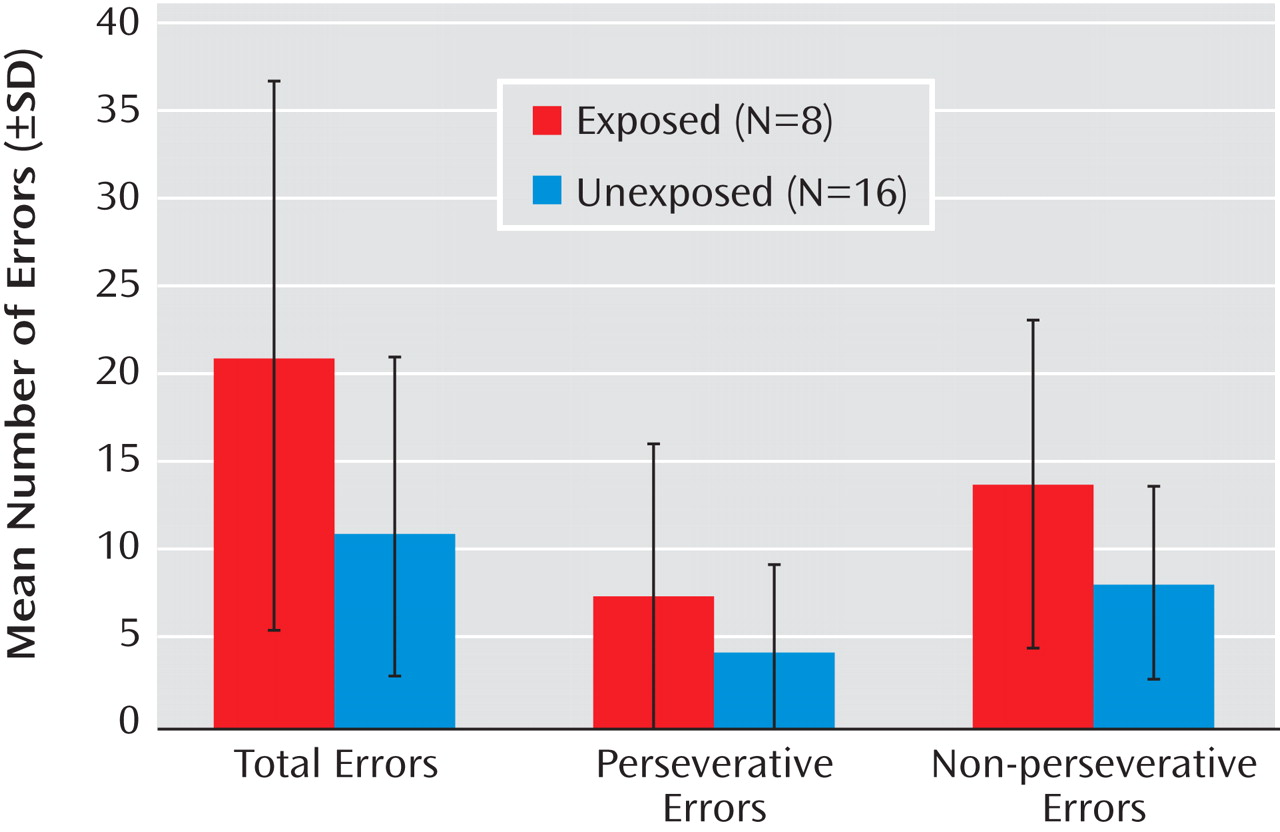
Case subjects exposed in utero to infection committed significantly more total errors than unexposed case subjects on the Wisconsin Card Sorting Test (
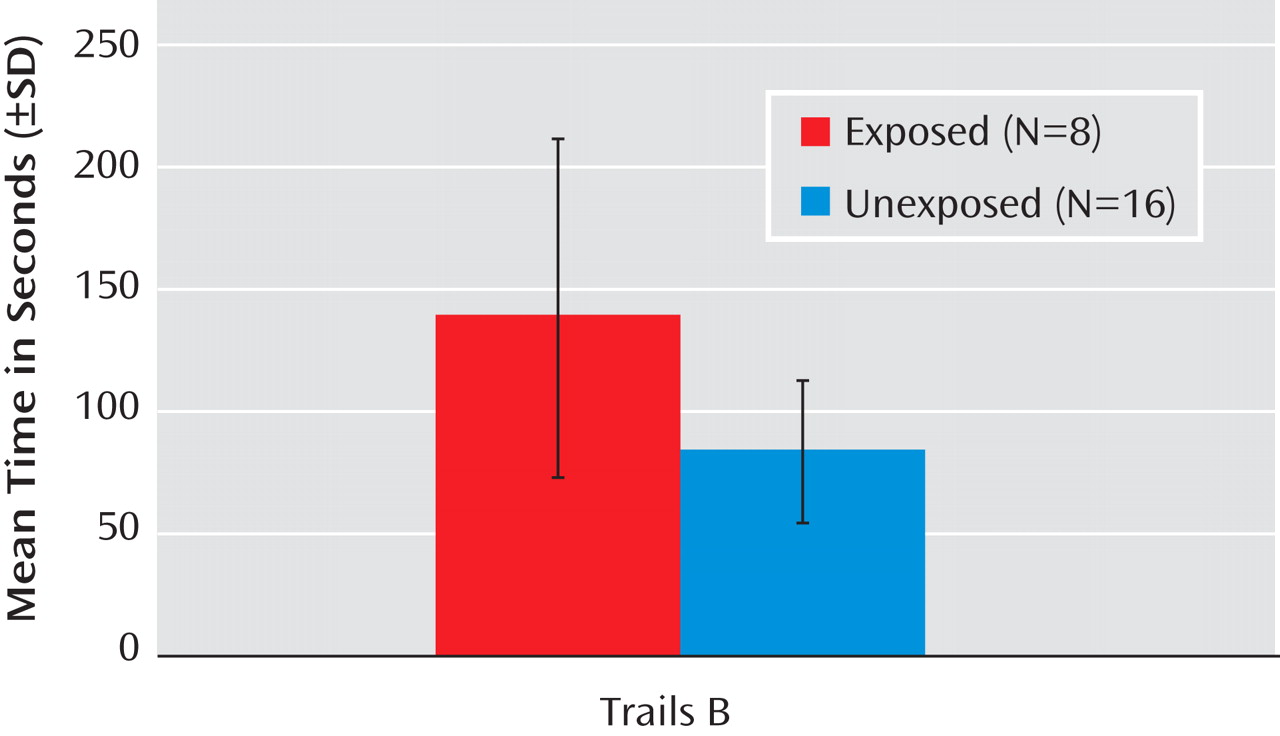
Figure 2 ) and took significantly more time to complete the Trails B (
Figure 3 ). However, there were no differences between exposed case subjects and unexposed case subjects on time to complete the Trails A, a measure of psychomotor processing speed (see
Table 3 ).
Comparison of Other Executive Function Measures Between Case Subjects Exposed and Unexposed to Prenatal Infection (Secondary Analyses)
Exposed and unexposed case subjects did not differ significantly in performance on any of the other neuropsychological measures except for figural fluency, which was significantly reduced for exposed compared to unexposed case subjects (p<0.001) (see
Table 3 ). Performance on digits backward and letter-number sequencing was poorer in exposed compared to unexposed case subjects, but these differences did not reach statistical significance.
The results were similar when the sample was restricted to case subjects with schizophrenia or schizoaffective disorder. Case subjects who were exposed to infection in utero evidenced more total errors (p<0.001) on the Wisconsin Card Sorting Test (mean=19.4 [SD=16.3]) compared to unexposed case subjects (mean=12.8 [SD=8.6]). Time to complete the Trails B was significantly longer (p=0.002) in the exposed (mean=153.4 [SD=64.1]) compared to the unexposed (mean=98.7 [SD=21.4]) case subjects. Time to complete the Trails A did not differ significantly between the exposed (mean=49.7 [SD=23.6]) and unexposed (mean=43.3 [SD=13.1]) case subjects. For the additional neuropsychological tests, the results for case subjects with schizophrenia and schizoaffective disorder were similar to those for the full schizophrenia spectrum disorders sample (data not shown).
Discussion
To our knowledge, these data represent the first demonstration of an association between prenatal infection and cognitive dysfunction in schizophrenia. Consistent with our hypothesis, case subjects who had serologically documented prenatal exposure to influenza or to elevated toxoplasma IgG antibody performed significantly more poorly than unexposed case subjects on the Wisconsin Card Sorting Test and on the Trails B. Strengths of the study include a well-characterized, representative cohort and direct, prospectively acquired biomarkers of infection.
As noted above, three previous studies have specifically examined the relation of obstetric complications to performance on the Wisconsin Card Sorting Test and Trails B. In the first study, schizophrenia case subjects with a history of obstetric complications committed more perseverative errors on the Wisconsin Card Sorting Test than those without obstetric complications
(13) . In the second study, obstetric complications were correlated with perseverative errors on the Wisconsin Card Sorting Test in healthy subjects
(15) . However, the types of prenatal complications included in the analyses of that study were heterogeneous and were based solely on medical records from hospitals, rather than on data from prenatal sera. Although no correlation was found between perinatal obstetric complications and Wisconsin Card Sorting Test performance in schizophrenia case subjects in the latter study, the patient sample consisted of only 10 subjects and thus had limited statistical power. In contrast, there were considerably more patients with schizophrenia in the present study. In a third study, a marked and statistically significant increase in the co-occurrence of poor Trails B performance and the presence of at least one obstetric complication was reported in schizophrenia case subjects relative to comparison subjects
(16) . While that study did not directly test for an association between perinatal obstetric complications and Trails B performance, the finding is consistent with the present results.
Examination of the pattern of results raises several questions. First, we consider whether the findings of impaired performance in exposed case subjects on the Trails B and other executive function measures can be attributed to a psychomotor processing speed deficit. This seems unlikely because we observed no difference between exposed and unexposed case subjects on Trails A time. A second question is whether the results might be explained by greater problems in abstraction and concept formation in exposed case subjects, as these neurocognitive abilities are required for Wisconsin Card Sorting Test performance; however, the Trails B test does not require concept formation.
Third, we consider an association between prenatal infection and working memory performance, a domain assessed in the secondary analyses. A statistical trend was observed for diminished performance on digits backward, suggesting a deficit in storage or manipulation of information in working memory. There was also a statistical trend for a decrement in performance on letter-number sequencing, a task that places greater demands on information manipulation in working memory than digits backward. No deficits were observed on digits forward, however, which tests simple short-term memory storage, or on the auditory n-back test, which is most strongly related to working memory updating. Hence, there are suggestions that certain components of working memory may have been disrupted by prenatal infection.
The findings of the secondary analyses were also somewhat inconsistent for fluency. Exposure to infection was associated with a significant decrement in performance on figural fluency and with a moderate though nonsignificant disruption in category fluency, but no group differences were observed in letter fluency.
Set shifting is a common neurocognitive function required for performance on both the Wisconsin Card Sorting Test and the Trails B. This function refers to the ability to switch mental sets in order to perform a given task. In contrast, the executive functions examined in our secondary analyses do not have a set-shifting component. Thus, these and previous findings
(15,
16) suggest that this neuropsychological function may be particularly vulnerable to the effects of prenatal and perinatal insults on the developing brain.
The relevance of these findings to the pathogenesis of schizophrenia is underscored by the fact that to date, there are no established etiologies of neurocognitive function in this disorder. There is, however, evidence that certain genetic polymorphisms may be related to several of the cognitive impairments observed in schizophrenia
(30,
31) . The present study suggests that environmental insults that occur during the prenatal period and that have been associated with risk of schizophrenia may similarly lead to cognitive vulnerability. Combined with preclinical studies, these results may lead to an improved understanding of the etiopathogenic pathways that account for disruptions in specific aspects of executive function and suggest potential strategies aimed at preventing and treating these impairments.
We note several limitations to this study. First, we used a proxy measure of influenza exposure; however, as previously described
(11), this measure was well validated against seroconversion. Second, given the modest sample size, it is possible that the absence of statistically significant associations between prenatal infection and certain executive function measures in our secondary analyses were due to insufficient statistical power; thus, larger samples will be necessary to confirm the present findings. Third, we were not able to assess whether the observed effects were specific to schizophrenia because of the lack of a sufficient number of serologically documented exposed healthy comparison subjects and the absence of a psychiatric comparison group.
Conclusions
We have demonstrated that prenatal infection is associated with considerably impaired performance on the Wisconsin Card Sorting Test and the Trail Making Test, part B, both of which have been shown previously to be related to perinatal obstetric complications. In contrast to those previous studies, the present study featured a well-characterized, representative birth cohort and direct, prospective biomarkers of infection. The pattern of findings suggests that cognitive set-shifting ability may be particularly vulnerable to this gestational exposure. Additional research is needed to assess whether these finding are also present in comparison subjects, to further examine specificity to these executive function measures, and to assess whether prenatal infection is associated with neuroanatomical or neurophysiological anomalies in schizophrenia that may be related to the observed disturbances. We also plan to examine additional types of obstetric complications to assess whether they have similar effects on neurocognitive and other outcomes, which may suggest shared or distinct underlying mechanisms.