The inability to ignore irrelevant noises in the environment is a common problem for people with schizophrenia. First described by Bleuler nearly 100 years ago, this flooding of sensory information can have a substantial impact on quality of life and may be related to disease pathology
(1,
2) . To better understand this core problem in schizophrenia, investigators have developed physiological and behavioral measures to study patients’ responses to sounds.
One commonly studied physiological measure is the P50 sensory gating paradigm, in which evoked responses to pairs of clicks are measured with EEG techniques. In healthy persons, responses to the second click in a pair of clicks are inhibited as part of a sensory gating or filtering mechanism
(3) . In persons with schizophrenia, responses to this repeated stimulus are not inhibited, which is interpreted as an inhibitory failure in sensory gating
(4) . In a recent functional MRI (fMRI) study
(5), we identified greater activation of the hippocampus, thalamus, and prefrontal cortex during a repeated-click sensory gating task in patients with schizophrenia. Other paradigms, such as the pre-pulse inhibition task, in which a weak stimulus preceding a startling stimulus diminishes the startle effect, have also been used in attempts to distill the sensory gating phenomenon into responses to pairs of stimuli
(6,
7) . The simplicity of these measures, particularly the ability to translate the tasks into animal models, has revealed valuable information about the neurobiology underlying deficits in sensory gating.
A weakness of such measures, however, is the unknown degree to which repeated clicks or stimuli in other modalities reflect the experience of people with schizophrenia in daily life, that is, an inundation by real noises in the environment. Previous studies using word stimuli or sounds from the environment have revealed behaviors that imply specific cortical deficits in schizophrenia. Since the late 1970s, studies using dichotic listening paradigms have shown that individuals with schizophrenia are more distractible when trying to perform other tasks, such as visual tracking
(8) and speaking
(9) . Other listening studies, using dichotic speech tasks, digit pair tasks, and monitoring tasks, have differed in their findings but have consistently demonstrated differences in the lateralization of auditory processing in schizophrenia
(10) .
Studies using more naturalistic or complex sounds have the advantage of higher face validity but have not been studied in the context of sensory gating. In this study, we sought to develop a new sensory gating task with higher face validity—one that more closely relates to real-life situations. The simple task involves passive listening to “urban white noise,” a mixture of common sounds from the environment simulating what a person may experience in a busy urban setting, including multiple conversations and noises recorded from a party, music, and conversations from radio broadcasts. The stimulus also includes items frequently reported to be noticed more often by people with schizophrenia, such as traffic noise and a refrigerator motor randomly cycling on and off. We tested the hypothesis that hemodynamic response in the hippocampus, thalamus, and dorsolateral prefrontal cortex, regions previously identified as exhibiting greater response during a repeated-click gating task, would be more active in subjects with schizophrenia during the urban white noise sensory gating task. We also evaluated the relationship between this new measure of sensory gating and typical P50 gating measures, and we describe subjective responses to the novel gating task by participants.
Method
Participants
The study included data from a total of 35 participants—18 outpatients with schizophrenia (7 women and 11 men; mean age=36.6 years [SD=12.0]) and 17 healthy comparison subjects (6 women and 11 men; mean age=36.7 years [SD=12.5]). Data from two other subjects were excluded because of excess head motion (>1 mm) during scanning. Diagnoses were confirmed with the Diagnostic Interview for Genetic Studies. No significant group difference in age was observed. Of the 18 participants with schizophrenia, 16 were treated with atypical antipsychotics, one with a conventional antipsychotics, and one with both conventional and atypical antipsychotics. The study was approved by the Colorado Multiple Institution Review Board, and all participants provided written informed consent.
fMRI Methods
After a hearing test (see below), a high-resolution T 1 -weighted three-dimensional anatomical scan was acquired for each subject for coregistration to functional data (inversion recovery-spoiled gradient-recall acquisition [IR-SPGR], repetition time=9 msec, echo time=1.9 msec, inversion time=500 msec, flip angle=10 degrees, matrix=256×256, field of view=220 mm 2, 124 coronal slices 1.7 mm thick). Functional images were acquired with a gradient-echo T 2 * blood-oxygen-level-dependent (BOLD) contrast technique (repetition time=14,000 msec [as a clustered volume acquisition of 2000 msec, plus an additional 12,000 msec silent interval], echo time=30 msec, field of view=220 mm 2, 6464 matrix, 31 slices 4 mm thick, no gap, angled parallel to the planum sphenoidale). Additionally, one inversion recovery-echo planar imaging (IR-EPI) volume (inversion time=505 msec) was acquired to improve coregistration between the functional and anatomical scans.
Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark). Auditory stimuli were presented via MR-compatible headphones (Resonance Technology, Inc., Northridge, Calif.), and MR-compatible goggles (Resonance Technology, Inc.) were used for visual stimuli. Motor responses for the hearing test were collected via a fiber optic response pad (Cedrus Corp, San Pedro, Calif.).
fMRI Paradigm
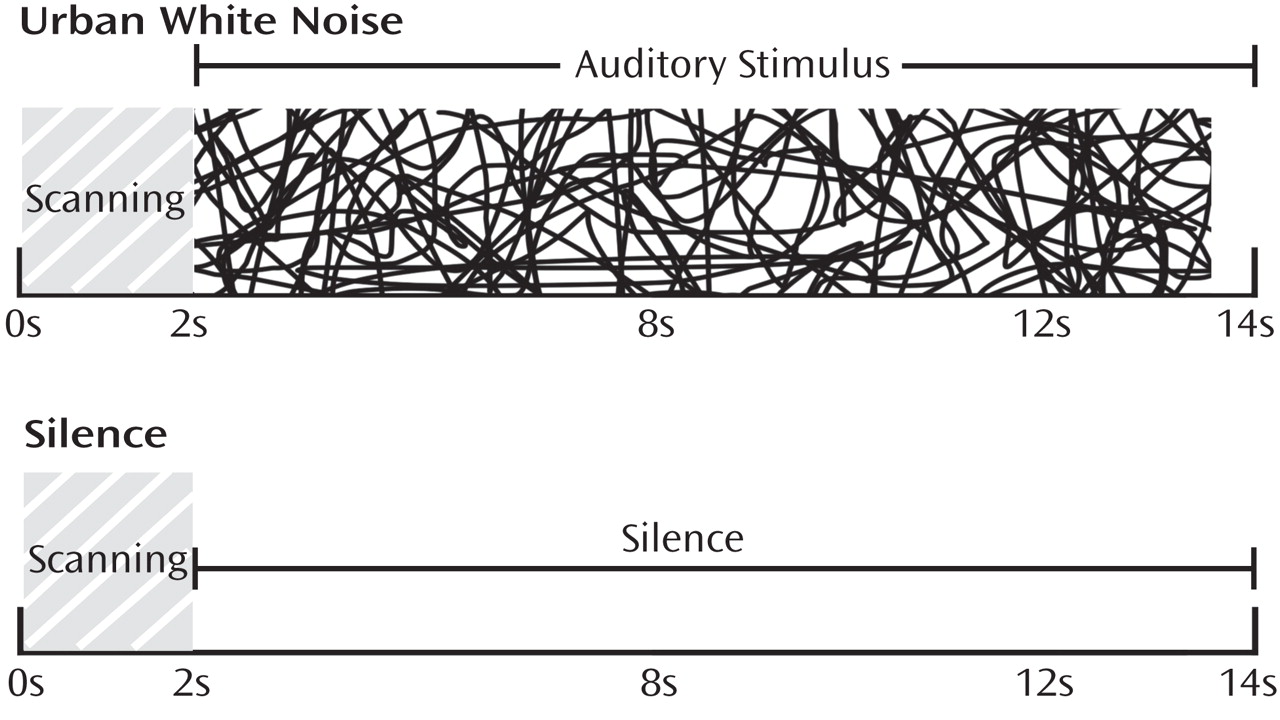
Prior to scanning, participants completed a hearing test in the scanner to set the task volume at 30 dB above hearing threshold. In the scanning environment, this sound level resulted in a clearly audible yet not overwhelming or startling volume. After the hearing test and structural scan, participants performed the sensory gating task while undergoing fMRI scanning. Participants watched a silent movie during the scan while auditory stimuli were played in the background. The study used clustered volume acquisition, in which scans are not continuously acquired but rather are spaced at long intervals, allowing stimuli to be presented in silence while still capturing the peak hemodynamic response. This technique has been shown to substantially improve signal detection in auditory tasks
(11) . For consistency with our prior sensory gating study using repeated clicks, the paradigm used a total repetition time of 14 seconds, which included an initial 2 seconds of scan acquisition followed by 11.5 seconds of either silence or the urban white noise stimulus (described below) (
Figure 1 ). Alternating 28-second blocks of silence and auditory stimuli were presented to the subjects over two runs, totaling 15 minutes. Between runs, brief conversations were held with participants to ensure that they were awake and responsive. After scanning, participants were asked to describe their experience with the open-ended question “What did you think about the task?”
The urban white noise stimulus consisted of a mixture of audio clips, which included segments from two talk radio shows; two classical musical pieces; sounds from a neighborhood block party with multiple background conversations; sounds of children playing; traffic sounds; a refrigerator motor cycling on and off; and frequent knocking sounds from glasses being set on countertops. The volumes of all of these elements were mixed so that no one element was readily identifiable. The subjective experience of the sound mixture was that of standing in a busy crowd of people in which multiple conversations were occurring, with a low level of indistinguishable background music and other sounds. A supplementary figure showing a power spectrum characterizing the stimulus is available in the data supplement that accompanies the online edition of this article.
fMRI Data Analysis
Data were analyzed using SPM2 (Wellcome Department of Imaging Neuroscience, London). Data from each participant were realigned to the first volume, normalized to the Montreal Neurological Institute (MNI) template using a gray-matter-segmented IR-EPI as an intermediate to improve registration between the EPI and IR-SPGR and smoothed with an 8-mm full width at half maximum Gaussian kernel. Data were modeled with a hemodynamic response function-convolved boxcar function, using the general linear model in SPM2. A 128-second high-pass filter was applied to remove low-frequency fluctuation in the BOLD signal. The primary analysis modeled stimuli as 28-second blocks of either silence or urban white noise. A secondary event-related analysis modeled each stimulus presentation separately to assess habituation effects within a block.
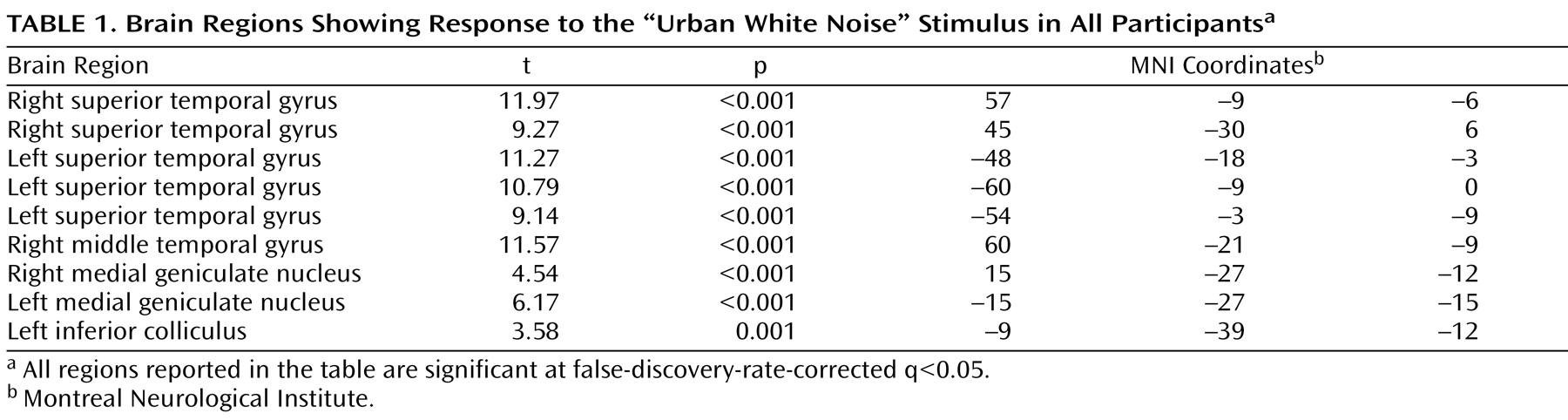
To account for both within-group and within-subject variance, a random-effects analysis was implemented. Parameter estimates for each individual’s first-level analysis (SPM contrast images) were entered into second-level t tests for each contrast of interest. The contrast urban white noise/silence was evaluated. The main effect of task was evaluated with a whole-brain analysis, corrected for multiple comparisons using the false discovery rate technique
(12) . For between-group comparisons, a priori hypotheses about activation in four regions, the superior temporal gyrus, the hippocampus, the thalamus, and the dorsolateral prefrontal cortex, were evaluated using anatomically defined regions of interest from Wake Forest University’s PickAtlas
(13) . The hippocampal and thalamic regions of interest included the entire anatomical structures. The dorsolateral prefrontal cortex region of interest consisted of Brodmann’s areas 9 and 46 combined, excluding the superior frontal gyrus. These regions of interest were identical to those used in our previous fMRI study of sensory gating using repeated clicks
(5) . The mean response for all voxels in each region of interest was determined using the MarsBar toolbox
(14) in SPM2. To improve statistical power, results were masked with a gray-matter mask consisting of the average gray matter from all participants obtained from their segmented IR-EPIs. Functional results were overlaid onto the group average T
1 -weighted anatomical images and thresholded at a whole-brain p<0.01 for visualization.
EEG Paradigm and Methods
Details of the paired-click recording paradigm have been described previously
(15) . The P50 potential was identified and measured by using a computer algorithm, also described previously
(15) . The amplitude of the P50 test wave was divided by the amplitude of the P50 conditioning wave, expressed as a percentage: the P50 ratio. Participants were given no special instructions concerning the clicks they were hearing. Recordings were obtained from 31 of the 35 subjects who were scanned. One participant with schizophrenia and three comparison subjects were unavailable for EEG recording. Data from one additional subject with schizophrenia were excluded because the minimum number of recorded responses (three sets of averaged evoked responses to 16 pairs of stimuli) was not obtained as a result of excessive eye blink and muscle artifact
(15) .
Results
fMRI
Passive listening to the urban white noise stimulus produced robust activation of the auditory pathway both in participants with schizophrenia and in healthy comparison subjects.
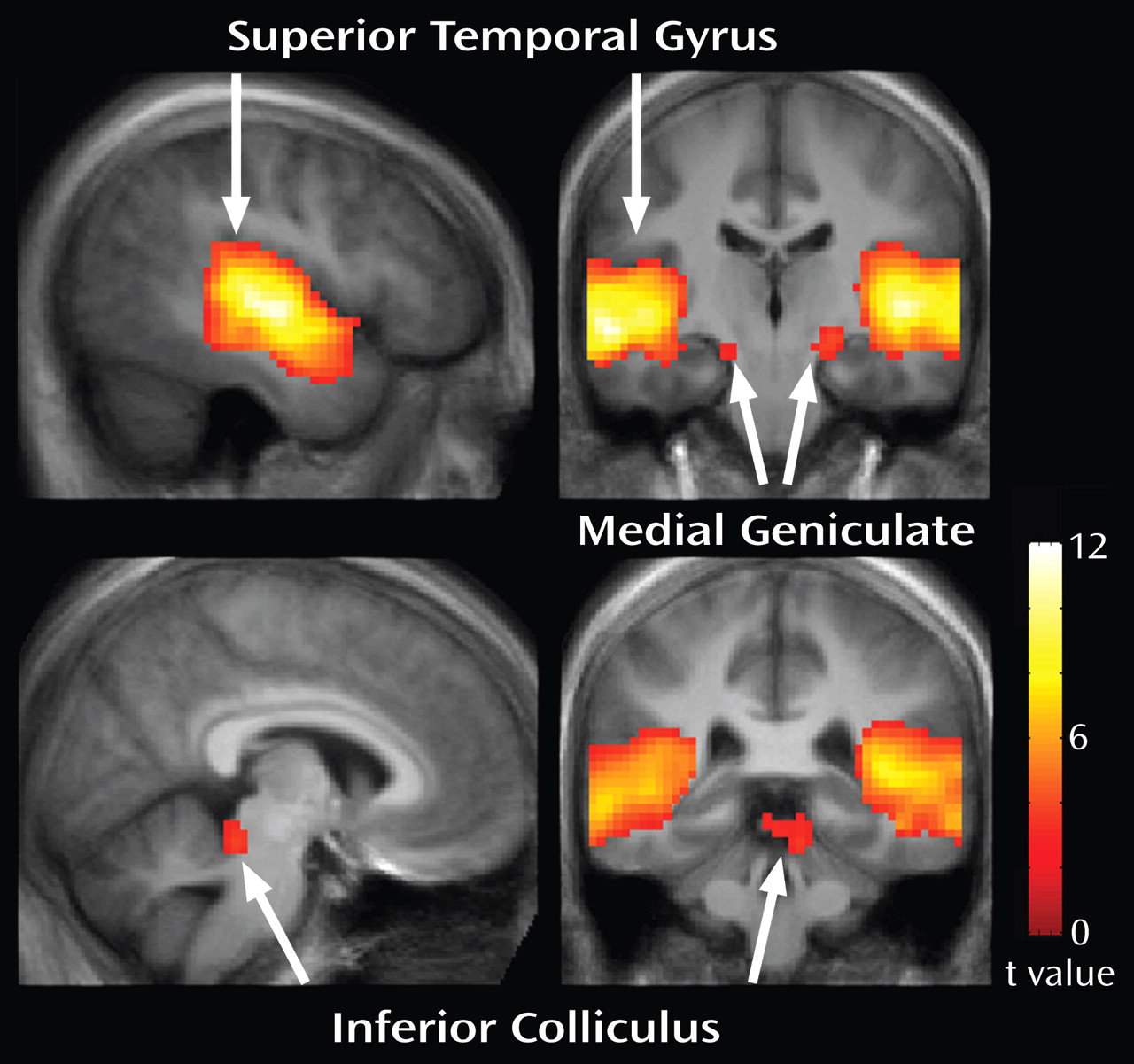
Figure 2 shows whole-brain responses to the stimulus across all participants, thresholded at a false-discovery-rate-corrected q<0.05. Activation was observed in the bilateral primary and secondary auditory cortices and medial geniculate nuclei. Activation of the left inferior colliculus also was observed. MNI coordinates for these regions are listed in
Table 1 .
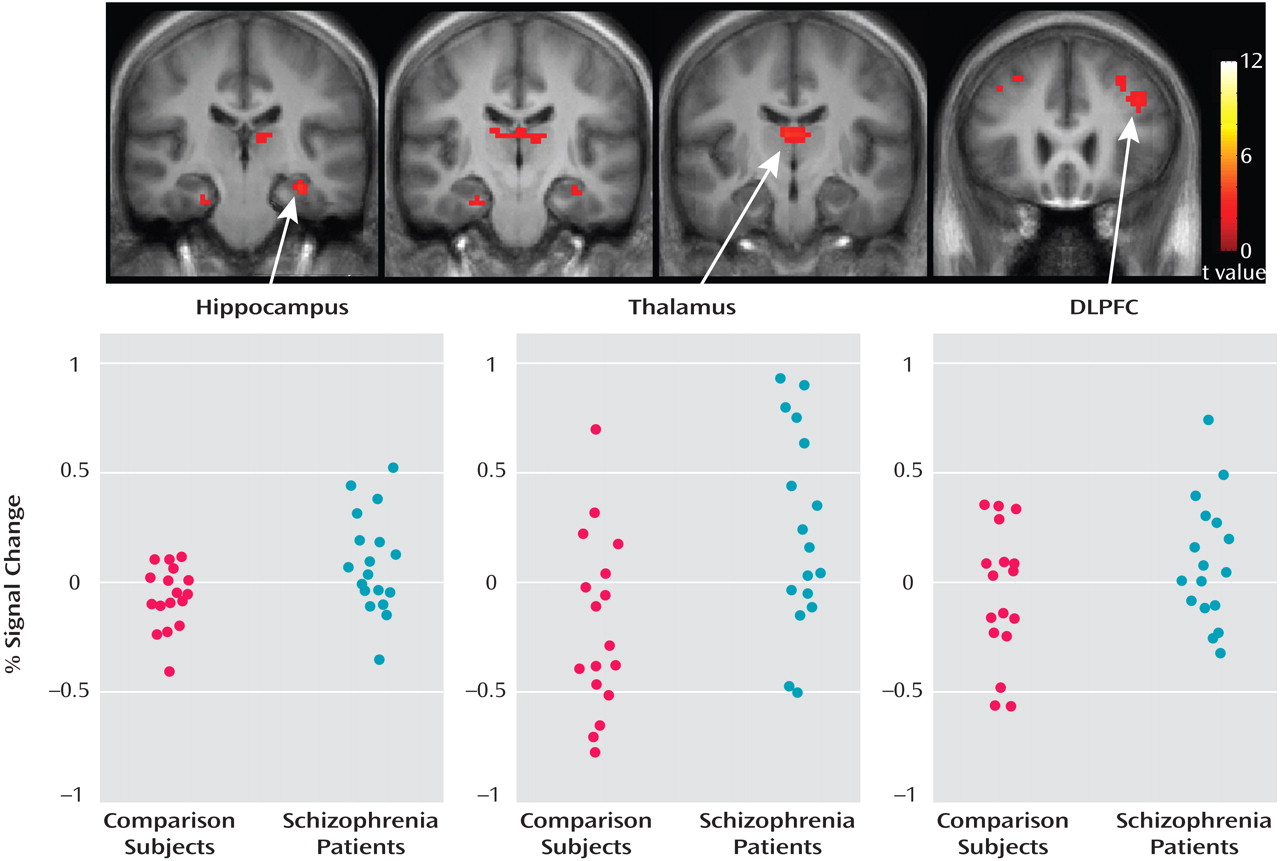
Figure 3 shows that responses were greater during the task in the schizophrenia group relative to the comparison group. Greater responses were observed in the hippocampus (left side: t=2.05, df=33, p=0.024), the thalamus (left side: t=1.95, df=33, p<0.030; right side: t=1.68, df=33, p=0.051), and the dorsolateral prefrontal cortex (left side: t=1.80, df=33, p=0.040). Differences in superior temporal gyri responses did not reach significance (right side: t=1.12, df=33, p=0.14; left side: t=1.23, df=33, p=0.11). Within the anatomically based regions of interest, local maxima were t=3.43 (x=3, y=–12, z=12) in the right thalamus; t=3.49 (x=–33, y=–21, z=–18) in the left hippocampus; and t=2.86 (x=–42, y=21, z=18) in the left dorsolateral prefrontal cortex. Individual participant responses for local maxima, in terms of BOLD percent signal change relative to the global mean, are shown in
Figure 3 .
To evaluate differences in responses within a block, individual stimulus presentations also were evaluated as events, separating responses from the first and second trials. In comparing the first stimuli with silence, no group differences were observed. In comparing the second stimuli with silence, greater responses in the hippocampus (t=1.42, df=33, p<0.082) and reduced responses in the superior temporal gyri (right side: t=1.37, df=33, p=0.090; left side: t=1.49, df=33, p=0.073) approached significance in the schizophrenia group relative to the comparison group. Responses in the dorsolateral prefrontal cortex were not significantly different between groups. A nearly significant interaction between trial (presentation 1 vs. 2) and group was observed in the left hippocampus (t=1.6, df=33, p=0.059).
EEG
The schizophrenia group had significantly higher P50 test/conditioning ratios than the comparison group (t=3.07, df=28, p=0.002). Mean P50 ratios were 0.63 (SD=0.16) for the schizophrenia group and 0.32 (SD=0.13) for the comparison group. P50 test/conditioning ratios were significantly correlated with the BOLD response in the fMRI sensory gating task in the left hippocampus (R 2 =0.32, df=29, p=0.001) and the left dorsolateral prefrontal cortex (R 2 =0.19, df=29, p=0.015) and approached significance in the left thalamus (R 2 =0.11, df=29, p=0.067).
Participant Self-Reports
Participant reports of their experience during the task revealed that those with schizophrenia were more often distracted or bothered by the noise stimulus. None of the comparison subjects reported being bothered by the stimulus, although two complained that the scanner noise was loud. Nine of the 18 participants in the schizophrenia group reported being bothered or highly distracted by the stimulus. These reports ranged from descriptions of the stimulus as “a bit bothersome” to being “very hard to ignore.” One schizophrenia subject reported hearing a “phone noise” during the task, although no telephone sounds were present in the stimulus. The most poignant comment came from a 44-year-old male participant with schizophrenia who commented, “I was trying to figure out where those little f—ers were coming from.”
Discussion
Brain hemodynamic responses to the complex noises used in this study were robust, including activation of the primary and secondary auditory cortices, the medial geniculate nuclei, and the inferior colliculus. Responses in the brainstem auditory nuclei and the medial geniculate nuclei were not expected, as more sophisticated experimental designs using cardiac gating typically are required to detect responses in these small structures
(16) . Detecting responses in these low-level auditory structures seems reasonable, however, given the stimulus energy and the broadband nature of the stimulus used.
Group differences in hemodynamic response during the sensory gating task included greater activation of the left hippocampus, the left and right thalamus, and the left prefrontal cortex in subjects with schizophrenia relative to comparison subjects. This finding is similar to results from our previous fMRI study using repeated clicks to study sensory gating
(5) . The largest observed group difference in response was greater activation of the hippocampus in the schizophrenia group. The hippocampus is morphologically and neurochemically altered in schizophrenia
(17) . Involvement of the structure in sensory gating deficits in schizophrenia had been proposed previously
(18) . While our previous study is the only fMRI report of direct involvement of the hippocampus in gating deficits in schizophrenia published to date, recent evidence from neurosurgical studies of patients undergoing invasive presurgical evaluation for epilepsy suggests involvement of the hippocampus in normal sensory gating
(19 –
21) . Animal studies have strongly implicated the involvement of the hippocampus in both normal and deficient sensory gating
(22 –
24) .
Greater responses during the sensory gating task also were observed in the thalamus in the schizophrenia group relative to comparison group. The thalamus plays a key role in gating information to the cortex, mediated by the nucleus reticularis, a thin layer of inhibitory neurons activated by g-aminobutyric acid (GABA)
(25) . Although early studies suggested involvement of the thalamus in auditory sensory gating, only recently has auditory gating been demonstrated in neurons in the nucleus reticularis of the thalamus
(23) .
Greater hemodynamic response in subjects with schizophrenia was also observed in the prefrontal cortex, which is consistent with our previous study using repeated clicks to assess gating
(5) . The prefrontal cortex has long been hypothesized to play a role in inhibitory processes such as sensory gating
(26) . Invasive recordings suggest that the prefrontal cortex plays a role in the early stages of the gating response
(19) . Recent magnetoencephalography studies further support prefrontal cortex involvement in gating responses, in both the auditory and somatosensory domains
(27) . The responses observed in the present study also may reflect differences in higher cognitive processes, such as attention, which typically are not thought to play a dominant role in early sensory gating. Self-reports indicated that many participants in our schizophrenia group found the noises to be overtly distracting, which suggests that additional cognitive resources, possibly including response of the prefrontal cortex, were engaged in these participants. Studies that modulate the level of distraction and control for attention are needed to further elucidate the role of the prefrontal cortex in sensory gating tasks.
The greater hemodynamic responses observed in this study are consistent with physiological models of an altered balance between excitatory and inhibitory neurotransmission in schizophrenia. It is possible, for example, that the greater responses in schizophrenia patients reflect an inhibitory deficit stemming from abnormal GABA neurotransmission, which has been proposed as a “final common pathway” for cortical dysfunction in schizophrenia
(28) . Such an inhibitory deficit may lead to the inappropriate excitation of a network of brain regions, as has been proposed previously
(5) . Alternatively, the increased responses observed in this study may stem from compensatory processes. Since no overt demands were made on participants during the fMRI task, however, it is difficult to speculate about what deficit, if any, would be compensated for in the schizophrenia patients.
The positive correlation between hemodynamic responses in the fMRI task and evoked responses during the paired-click sensory gating paradigm suggests that the urban white noise sensory gating paradigm may at least partially tap into neurobiological processes involved in the gating mechanisms studied previously. It is also possible, however, that the correlation between these phenomena are mediated by a common underlying biological factor not directly measured. The modest correlation coefficients observed are not unexpected given the substantial difference in both the paradigms used and the responses measured. Typical P50 auditory gating tasks, which are thought to be largely preattentive, record responses to discrete stimuli at a 50-msec latency. The urban white noise fMRI task is not a discrete stimulus (it lasts several seconds), and it incorporates both early responses, such as the P50, and later responses, which are known to be more dependent on additional cognitive processes.
There are several limitations to this study. All participants with schizophrenia were under treatment with antipsychotics, which may affect their responses or the measured BOLD signal. Braus et al.
(29) showed that antipsychotic treatment, particularly with conventional agents, may alter the BOLD response in some brain regions. More recent studies, however, have not shown medication effects on the BOLD response in the context of bipolar disorder
(30) or schizophrenia
(31) . Another limitation of this study is the use of silence as a baseline comparison. Because resting state activity may be altered in schizophrenia
(32), studies using graded auditory stimuli or other control conditions are necessary. An additional caveat is that the open-ended question we used to elicit the self-reports described here lacked structure and hence may have low sensitivity in capturing the salient aspects of participants’ experiences.