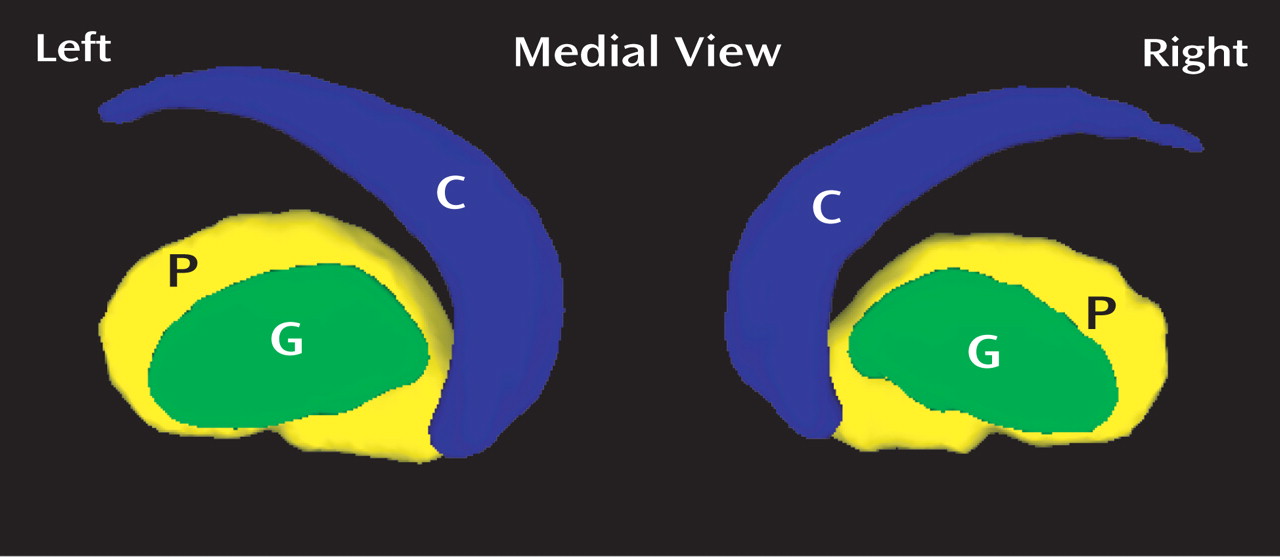
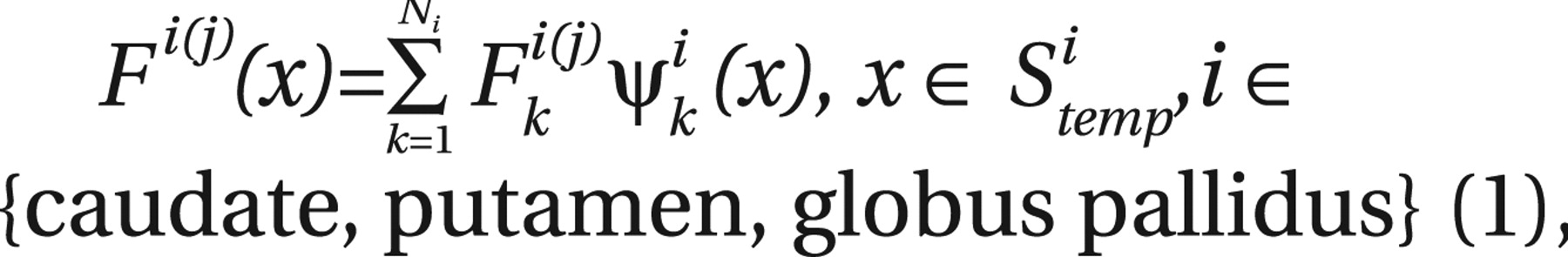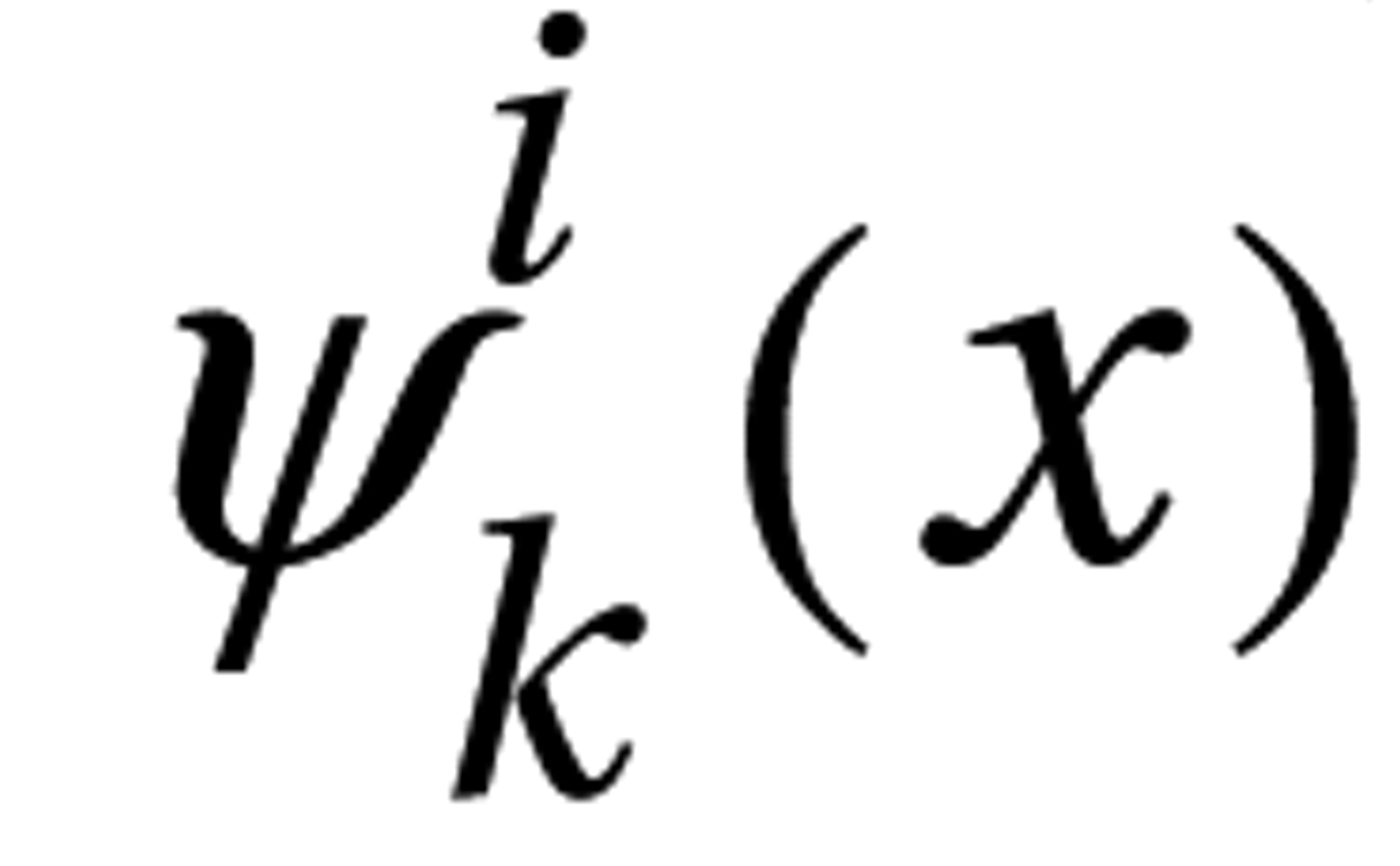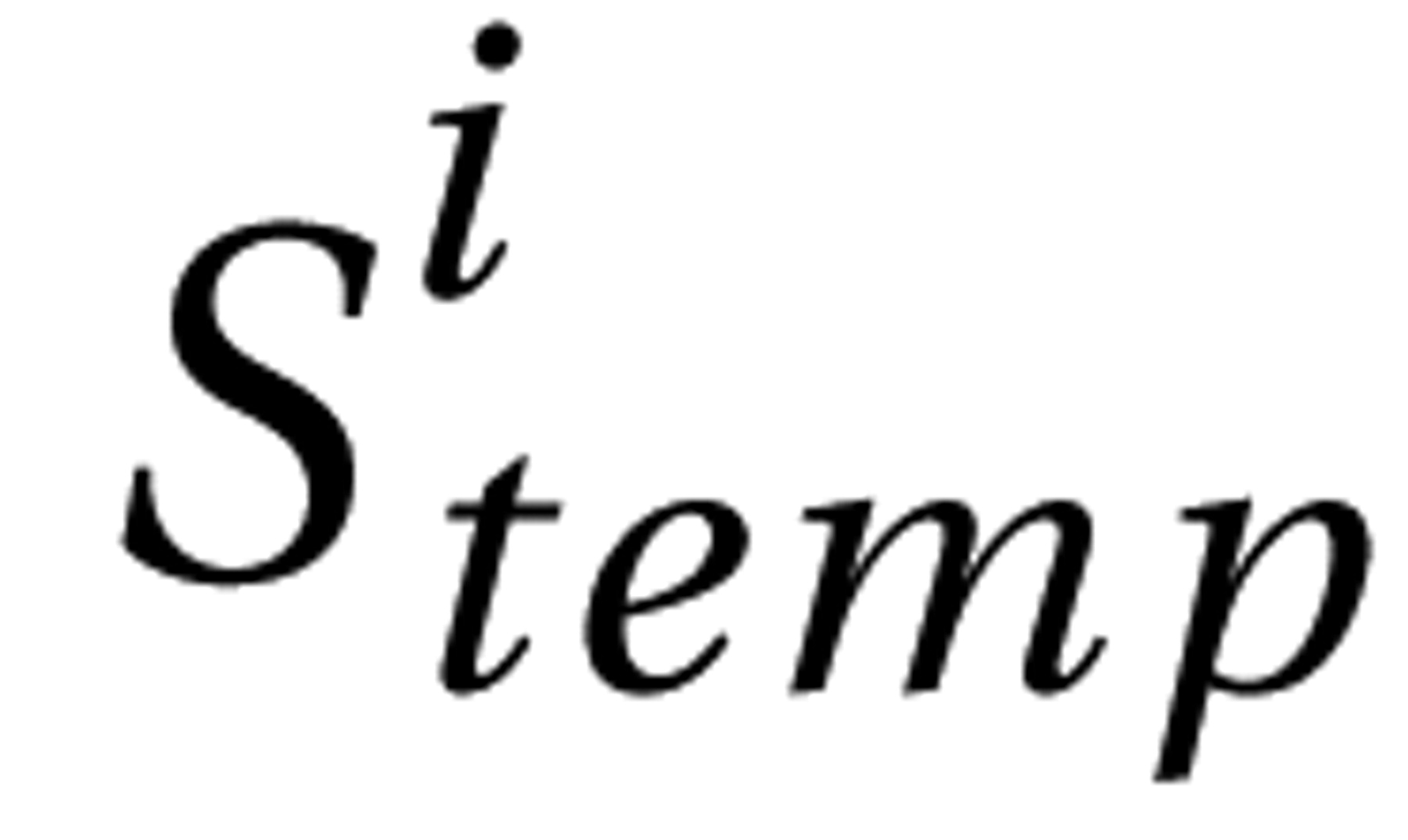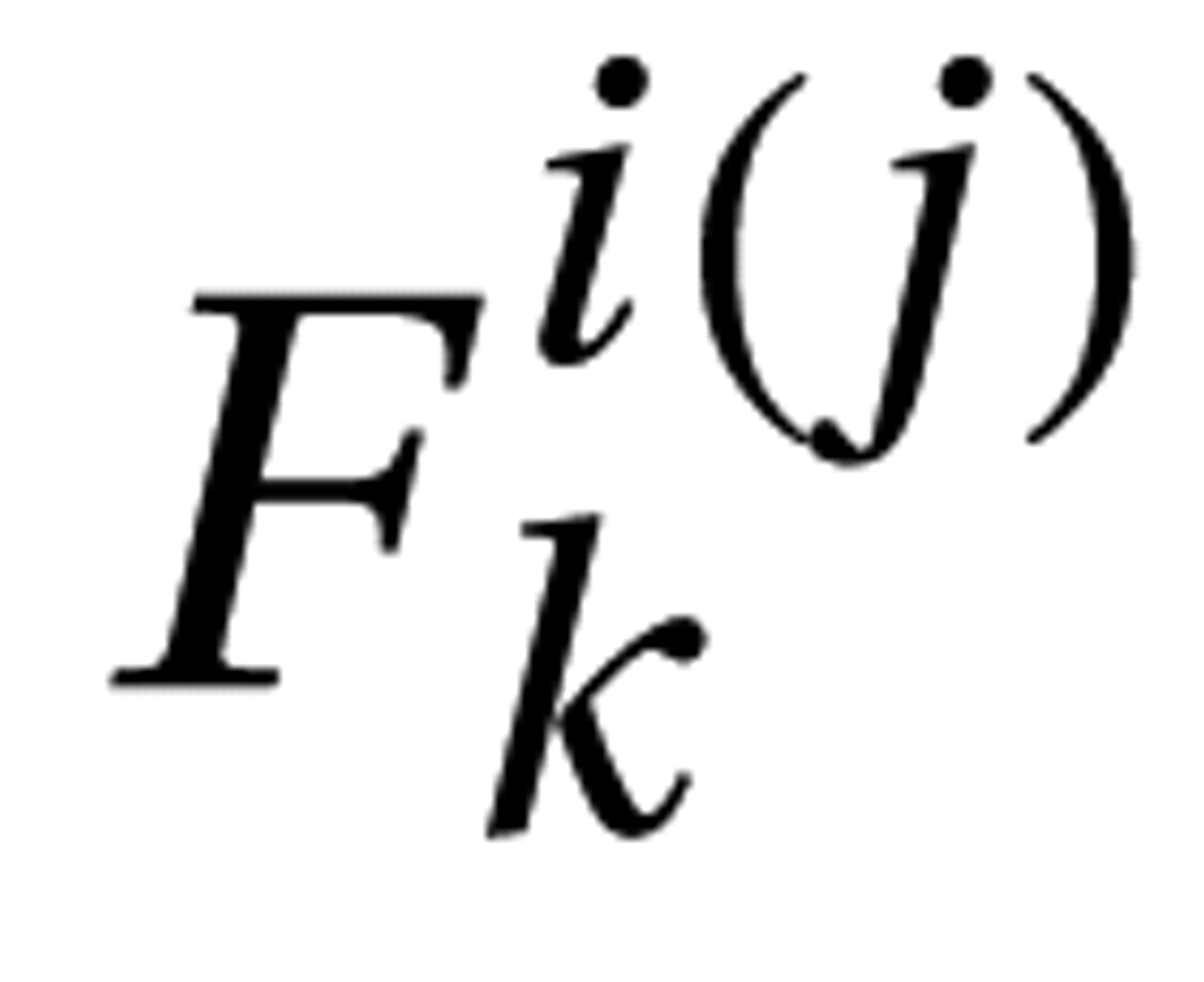Attention deficit hyperactivity disorder (ADHD) is a prevalent neuropsychiatric syndrome characterized by excessive difficulty with inattentive and/or hyperactive-impulsive behavior. Before the era of neuroimaging, neurologists reported colocalizing motor impairments and cognitive anomalies that implicated frontal lobe subdivisions (and/or interconnected subcortical regions “in circuit” with the frontal lobe) as candidate sources of developmental delay/deviation
(1) . Among these subcortical regions, the basal ganglia structures have been particularly emphasized because they are important for selecting appropriate goal-directed behavior
(2) . Anomalous basal ganglia structure and function may thereby contribute to difficulty with response control that is characteristic of ADHD
(3,
4) .
With the advent of magnetic resonance imaging (MRI), investigators began using MR-based volumetric techniques to study basal ganglia structures in ADHD
(5,
6) . These studies, yielding volumetric measurements of the caudate, putamen, and globus pallidus, have produced conflicting results. Decreased right caudate volume has been reported in prior studies
(7 –
9) and in a meta-analysis
(10) . Reversed caudate asymmetry, i.e., not the typical right-left caudate relationship, was reported in four other publications
(11 –
14) . Decreased volume of the putamen was reported once
(15), and reversed putamen asymmetry was reported once
(16) . Decreased volume of the globus pallidus has been reported twice on the left
(7,
17,
18) and twice on the right
(14,
15) . These discrepancies may reflect the underlying biological heterogeneity in ADHD, including sex-specific effects. Discrepancies may also be a consequence of limitations in anatomic imaging methods.
Investigations of basal ganglia structures have thus far been limited to examination of volumetric analysis and are prone to missing localized abnormalities. Large deformation diffeomorphic metric mapping (LDDMM)
(19) is a powerful computational tool that provides detailed analysis of the morphological shape of cortical and subcortical brain regions, thereby allowing for precise examination of these structures, well beyond what has been examined in prior MRI studies of ADHD. Thus far, LDDMM has widely been used to study neurodegenerative diseases and neuropsychiatric disorders, such as Alzheimer’s disease and schizophrenia; it has revealed specific regional shape changes associated with stages of diseases
(20,
21) .
In ADHD, LDDMM offers a critical opportunity to precisely localize differences within the basal ganglia. Furthermore, it can be used to determine the nature of those differences, both in terms of direction (inward or outward deformation) and degree. In doing so, it is possible to both 1) further specify ADHD-associated differences in brain structures observed using volumetric techniques and, more important, 2) detect localized differences within brain structures that have thus far gone unrecognized (because localized effects were “washed out” in examination of total volume).
In this study, we further specify volumetric differences and use LDDMM to identify local shape differences in the basal ganglia in children with ADHD. Furthermore, we sought to examine the interaction between ADHD and sex on differences in basal ganglia volume and shape. Based on the prior findings from our study revealing multiple frontal circuit involvement at the cortical level, with reductions in both premotor and prefrontal cortical volumes
(22), we hypothesized that children with ADHD, particularly boys, would show mild reductions in basal ganglia volume, with remarkable nonuniform shape differences distributed over the basal ganglia. To achieve this, LDDMM
(19,
23 –
25) was used to map basal ganglia shapes in populations of typically developing children and children with ADHD. The outcome of LDDMM, a surface deformation map indexed over a template coordinate system of the basal ganglia, encodes variations of local shape differences of each individual subject relative to the template, which facilitates statistical inference to investigate shape differences across groups. Thus, in this article, we first report the template generation for the basal ganglia structures using a subset of our typically developing subjects. The templates were then used as references to characterize local shape variation of each individual subject using LDDMM for statistical testing on shape differences between the groups of typically developing children and children with ADHD.
Results
Demographic Information
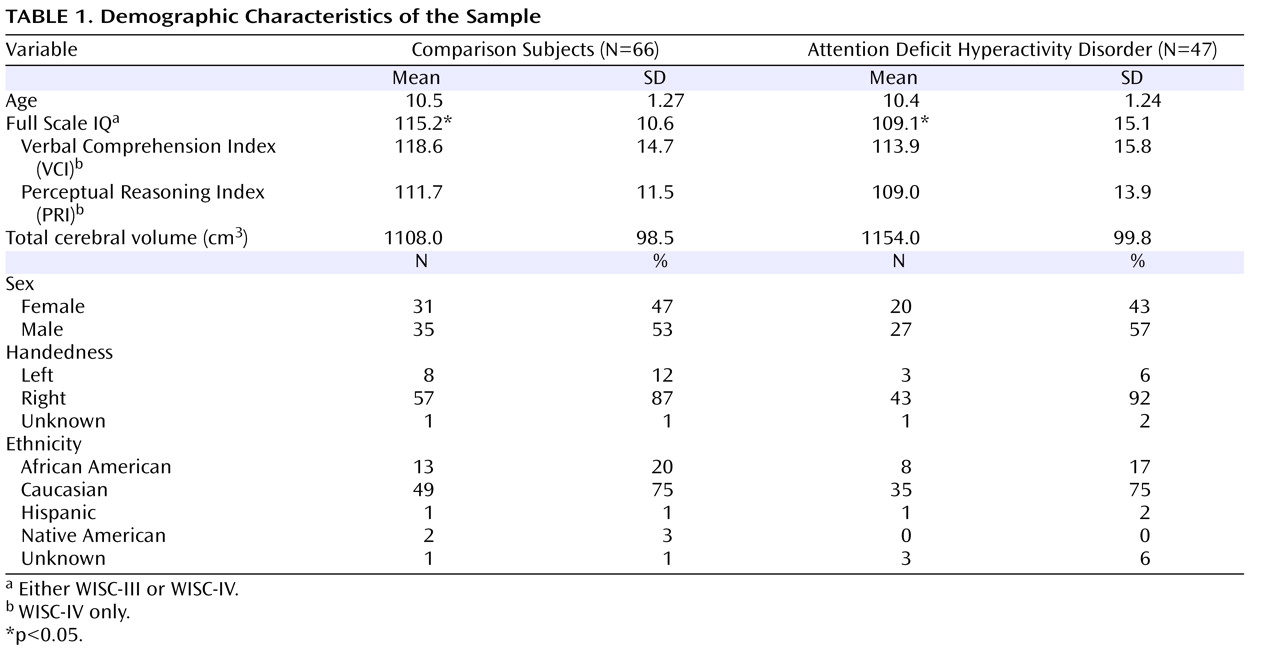
Demographic information for the sample is provided in
Table 1 . Participants were 75% Caucasian, 18% African American, 2% Hispanic, 2% Native American, and 3% unspecified. ADHD and comparison groups did not differ significantly in sex distribution (χ
2 =0.70, df=1, p=0.64), handedness (χ
2 =0.36, df=1, p=0.32), racial distribution (χ
2 =0.49, df=4, p=0.42), or age (F=0.05, df=1, 111, p=0.82).
There was a significant group difference in Full-Scale IQ, with ADHD children having lower Full-Scale IQ (mean=109.1, SD=15.1) than children in the comparison group (mean=115.2, SD=10.6) (F=6.32, df=1, 110, p=0.01). Full-Scale IQ scores include measures of working memory and processing speed. These skills are often impaired in individuals with ADHD, selectively lowering Full-Scale IQ scores in children with ADHD
(40) . For most children in this study (N=101), Full-Scale IQ was assessed using the WISC-IV, which provides for Verbal Comprehension Index (VCI) and Perceptual Reasoning Index (PRI) scores that are distinct from indices of working memory and processing speed. Thus, WISC-IV VCI and PRI scores were examined as more valid measures of intellectual reasoning in children with ADHD. There were no statistically significant differences between groups for either PRI or VCI score (PRI: F=1.11, df=1, 110, p=0.29; VCI: F=2.31, df=1, 110, p=0.13). Verbal and nonverbal intellectual reasoning were therefore felt to be equivalent in both groups. Nevertheless, to ensure the validity of our findings, Full-Scale IQ was entered as a covariate in group comparisons of the basal ganglia structures.
Total cerebral volume (TCV) for each subject was assessed using Freesurfer
(41) . Consistent with prior studies
(7,
12,
22,
42), the ADHD group showed decreased TCV compared to typically developing comparison subjects (ADHD, 1108 cm
3 ; typical, 1154 cm
3, F=5.922, p<0.07 in our study). TCV was also entered as a covariate in group comparisons of the basal ganglia structures.
Basal Ganglia Volumes
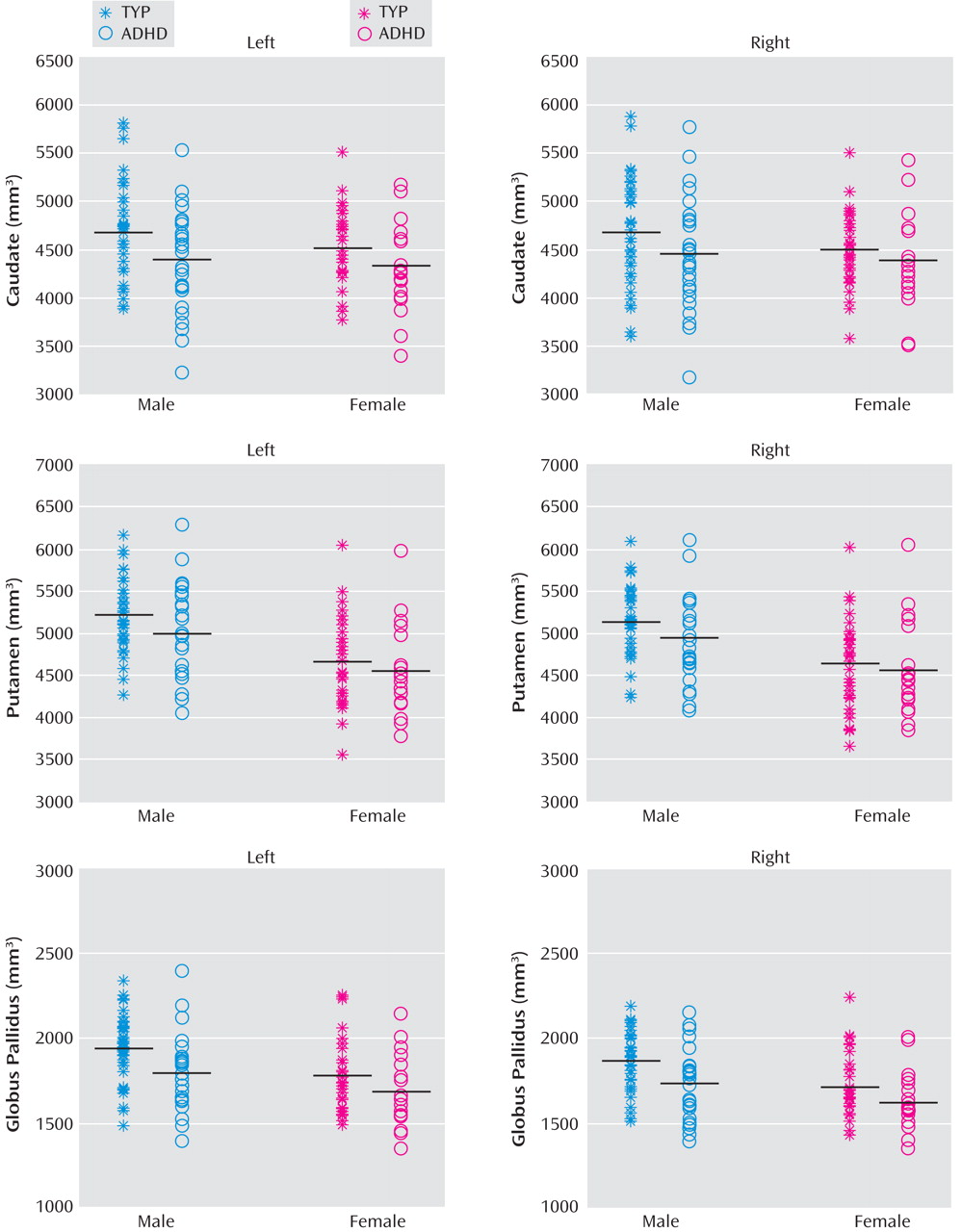
Linear regressions were used to explore diagnostic and sex effects on the volume of each structure. After control was added for TCV and Full Scale IQ, analyses for effects of diagnosis (displayed in
Figure 2 ) revealed smaller left basal ganglia volumes in children with ADHD compared to typically developing children in the caudate (p=0.03), putamen (p=0.03), and globus pallidus (p=0.004). For the right basal ganglia, significant differences were limited to the putamen (p<0.05) and globus pallidus (p=0.002). Significant sex effects were seen in the putamen bilaterally (both left and right, p<0.001) and globus pallidus bilaterally (both left and right, p<0.001). No significant interaction between diagnosis and sex were observed for any of the right or left basal ganglia structures.
To further investigate the diagnostic effects, we repeated the previous statistical testing separately for boys and girls. A significant diagnostic effect was seen for volumes of the left caudate, putamen, and globus pallidus (p<0.04, 0.004, 0.003, respectively) in boys but not girls after controlling TCV and Full-Scale IQ. In the right hemisphere, significant diagnostic effects were observed for volumes of the putamen (p=0.008) and globus pallidus (p=0.003) in boys but not girls after control was added for TCV and Full-Scale IQ.
Analyses examining differences based on ADHD subtype (controlling TCV, sex, and Full-Scale IQ) revealed smaller left basal ganglia volumes (caudate: p=0.03; putamen: p<0.02; globus pallidus: p=0.005) and smaller right putamen (p<0.05) and globus pallidus (p=0.002) in children with combined subtype ADHD compared to typically developing children. Compared with typically developing children, children with inattentive subtype ADHD showed smaller left caudate (p=0.03) and left and right globus pallidus (left: p=0.02, right: p<0.05) volumes. No group differences in the basal ganglia volumes were found in analyses comparing the combined and inattentive subtypes of ADHD.
Basal Ganglia Shapes
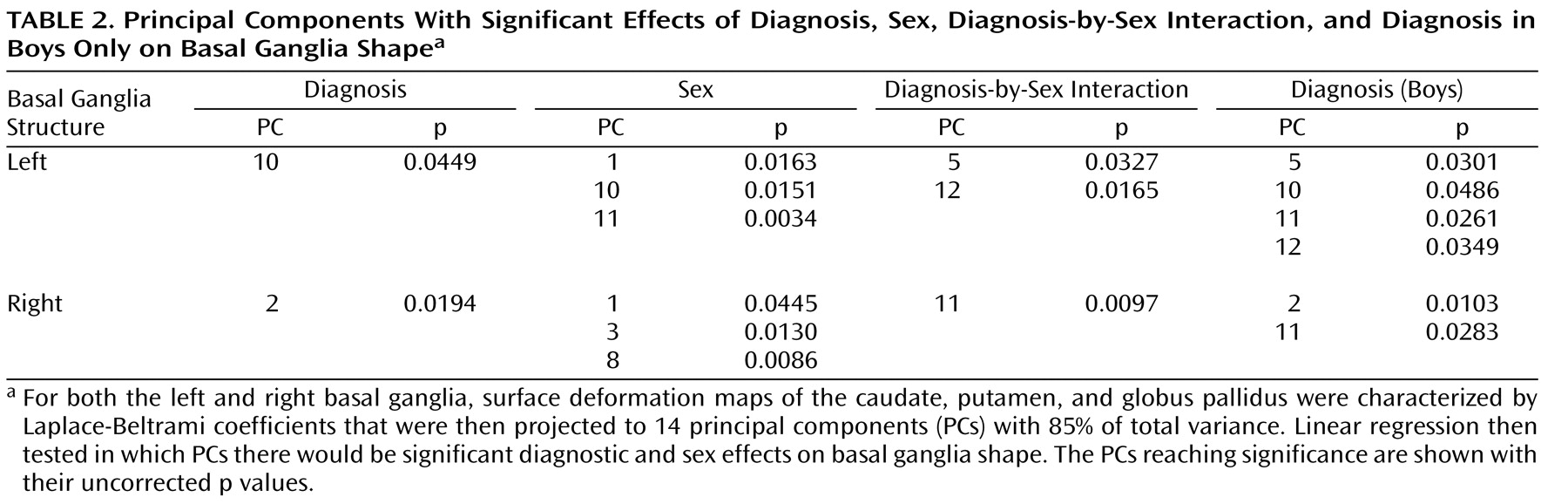
The surface deformation maps of the left caudate, putamen, and globus pallidus, respectively, were represented by 20, 18, and 8 Laplace-Beltrami coefficients based on the goodness of fit at a discrepancy level of 0.05. These Laplace-Beltrami coefficients were then projected to 14 principal components with 85% of total variance using PCA (see data supplement material for the variance contribution of the principal components). Linear regressions were used to test each of the principal component scores with diagnosis and sex as main factors controlling for TCV and Full-Scale IQ. Principal components reaching significance (p<0.05) are listed in columns 2–4 of
Table 2 (uncorrected p value), which indicate significant effects of diagnosis, sex, and their interaction on the basal ganglia shapes.
Linear regressions further examined diagnosis effects on the basal ganglia shapes in boys and girls separately when controlling for TCV and Full-Scale IQ. Analyses did not reveal significant diagnostic effects on left basal ganglia shapes within girls. Conversely, compared with typically developing boys, boys with ADHD showed significant diagnostic effects that are characterized by the 5th, 10th, 11th, and 12th principal components (the fifth column in
Table 2 ). Their visual representation is shown in
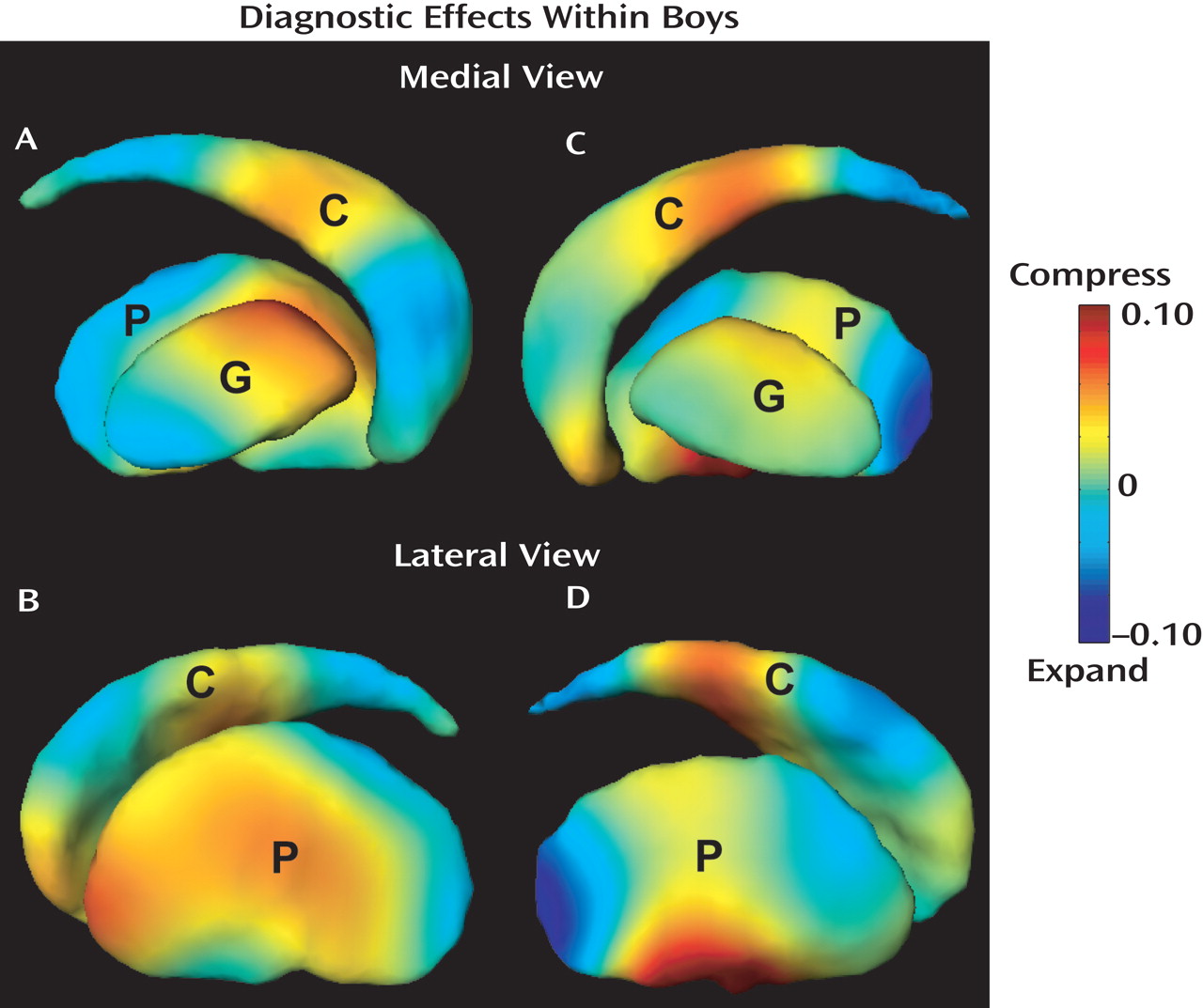
Figure 3 (A and B), which reveals a multifocal pattern of significant shape differences. Compared with typically developing boys, boys with ADHD showed significant volume loss (compression) in the left medial/lateral caudate body and anterior-lateral caudate head, left dorsomedial as well as in the left anterior/middle lateral putamen and anterior-medial globus pallidus. Boys with ADHD also showed significant expansion in the left medial caudate head, medial/lateral caudate tail, medial/lateral posterior putamen, and posterior-medial globus pallidus.
The surface deformation maps of the right caudate, putamen, and globus pallidus were characterized by 19, 18, and 7 Laplace-Beltrami coefficients. Fourteen principal components were extracted to represent 85% of total variance among these Laplace-Beltrami coefficients using PCA. Linear regressions were used to test each of the principal component scores with diagnosis and sex as main factors controlling for TCV and Full-Scale IQ. Principal components reaching significance (p<0.05) are listed in columns 2–4 of
Table 2, which indicate significant effects of diagnosis, sex, and their interaction on the basal ganglia shapes. Diagnostic effects on right basal ganglia shapes were also examined within boys and girls separately. Again, our analysis did not reveal significant diagnostic effects on right basal ganglia shape in girls; however, significant effects were found in boys. The map of right basal ganglia shape alteration in the boys with ADHD, shown in
Figure 3 (C and D), revealed a multifocal pattern of significant shape differences. Similar to findings for the left caudate, boys with ADHD showed compression, compared with typically developing controls, in the right medial caudate head and medial/lateral caudate body. Volume compression in ADHD boys was also seen in the right anterior-medial and medial/lateral ventral putamen. In contrast, expansion was seen in the right dorsolateral caudate body, medial/lateral caudate tail, and posterior putamen. The right globus pallidus showed relatively mild shape differences in the boys with ADHD.
Linear regressions did not reveal shape alterations in the basal ganglia in either combined or inattentive subtype of ADHD when compared with typically developing subjects, potentially due to small sample size.
Discussion
Neuroimaging studies of the basal ganglia in ADHD have previously reported findings based on volumetric analysis; in this study investigation with use of the LDDMM provided the ability to detect localized differences in shape of the basal ganglia. As a starting point, conventional volumetric measurements revealed ADHD-related anomalies in bilateral subdivisions of the basal ganglia in boys, but not girls. LDDMM methodology refined ADHD-associated differences (boys only) to a set of “compressions” (i.e., surface inward deformations) and expansions (i.e., surface outward deformation), such that LDDMM revealed multifocal shape differences of the basal ganglia in boys with ADHD when compared with typically developing boys. The shape analysis sheds light on the growing discrepancy in the literature between studies that have found differences and those that have not
(7 –
10) . Findings of expansion and compression distributed over the basal ganglia surface may help to explain findings of no volume differences reported in several prior studies of ADHD
(14,
17,
18,
43) .
Identification of local shape differences using LDDMM may help to specify the contributions of frontal-subcortical circuits to the pathophysiology of ADHD. Recent findings generated from nonhuman primate studies using striatal injection of retrogradely transported tracer
(44 –
46), as well as those from diffusion tensor imaging (DTI) studies of humans, have provided increasingly detailed mapping of cortical-striatal projections. While the segregation of functionally relevant motor, premotor and prefrontal circuits is not complete, revealing cascading interactions at the level of the striatum
(45,
46), the mapping provides some basis for interpreting the LDDMM findings in the context of specific frontal-subcortical circuits.
The locations in the basal ganglia showing surface inward deformation (compression deformation) in boys with ADHD included 1) bilateral anterior putamen, which is principally in circuit with rostral premotor areas (presupplementary motor area [pre-SMA], dorsal premotor area, frontal eye field, and supplementary eye field) and parietal association cortices
(44 –
46) ; 2) bilateral caudate head, which is in circuit with dorsolateral prefrontal (Brodmann’s areas 9 and 46) and orbitofrontal cortices
(44 –
46) ; 3) bilateral mid-caudate body, which is in circuit with rostral premotor and dorsolateral prefrontal regions
(44 –
46) ; 4) right ventral putamen, in circuit with the anterior cingulate
(45,
46) ; and 5) the left anterior globus pallidus, constituting outflow from left caudate and anterior putamen and, therefore, in circuit with prefrontal and premotor projections
(47 –
49) . These observations that basal ganglia regions with compression deformation in boys with ADHD fall within premotor, oculomotor, and cognitive (prefrontal) control circuits are in agreement with previous cortical findings
(22,
50) revealing that ADHD-associated structural and functional deviations from typical development are not restricted to any one frontal-striatal control loop.
Compared with typically developing boys, boys with ADHD also showed surface outward deformation (expansion deformation) in bilateral posterior putamen, left posterior globus pallidus, and right caudate tail. While not all of the implications of these ADHD-related bulges are clear, the posterior putamen findings are concordant with frontal-cortical thickness analysis of ADHD showing normal timing of maturation in the motor cortex, i.e., motor cortex “sparing” from delayed maturation, documented in the data on ADHD reported by Shaw and colleagues
(50) . Although speculative, it is tempting to wonder whether the early hyperactivity of young boys with ADHD activates (and thus facilitates) the spared maturation of motor cortex and SMA, thus paradoxically outpacing the other, delayed frontal regions that typically rein in motor hyperactivity. In our samples, including boys with and without ADHD, parent ratings on the CPRS-R Hyperactivity Scale were not correlated with the expansion deformation in the left posterior putamen (Spearman’s r=0.066, p=0.69), although there was a modest tendency for a significant correlation with expansion deformation in the right posterior putamen (Spearman’s r=0.227, p=0.09). A similar pattern in the correlation was observed when these analyses were restricted to boys with ADHD (left: Spearman’s r=0.316, p=0.13; right: Spearman’s r=0.363, p=0.08). Larger sample sizes will be needed to further investigate this hypothesis.
From the above discussions, the structural shape abnormalities of the basal ganglia in boys with ADHD suggest structural and functional deviations from typical development associated with ADHD are not restricted to any one cortical-subcortical control loop. This establishes precedence for future investigations examining differing preponderances between (and within level of) parallel loops as potential neural underpinnings of the clinical (and/or etiologic) heterogeneity of children receiving the ADHD diagnosis. Findings from functional imaging indicate that task-dependent contributions of individual premotor and prefrontal circuits to response control, impairments which appear central to ADHD, depend on conditions necessary for guiding goal-directed behavior
(51 –
53) . By examining correlations of brain shape abnormalities with indices of response control, specific regions and circuits contributing to the pathophysiology of ADHD can be better defined and understood.
No findings of volume and shape differences in the basal ganglia were revealed in girls with ADHD when compared with typically developing girls. This may suggest different underlying neuropathophysiologic processes in boys and girls with ADHD. Alternatively, the relative paucity of findings among girls with ADHD in cortical and subcortical structure
(18) may indicate that the core neuroanatomic abnormalities lie elsewhere in girls with ADHD. There is increasing evidence that reciprocal frontal-posterior cortical projections are critical to control of intentional movement
(54) and higher order executive processes
(55,
56) . It may be that abnormalities in white matter central to the pathophysiology of ADHD with downstream changes in cortical/subcortical structure are modified by X-linked or sex chromosome effects. Investigations using DTI would help to examine this hypothesis.