Several studies have examined changes in brain activity associated with improved performance on attentional and inhibitory tasks following administration of stimulant medication in both children
(1,
8 –
12) and adults
(13 –
15) with ADHD. These studies have varied considerably in design, sample characteristics, and behavioral paradigm used during scanning. They have typically studied small numbers of children with ADHD, and their findings have been highly variable, involving multiple regions across the brain, including the cerebellar vermis
(11), the basal ganglia
(1,
8), and the cerebral cortex
(1,
11) .
A better understanding of the neural mechanisms underlying the therapeutic actions of these medications may provide important insight into the pathogenesis of ADHD and promote the development of improved treatments. Clarifying the effects of stimulants on brain functioning in ADHD requires identifying the brain regions that function abnormally without medication and change in response to the medication. The degree to which brain functioning changes in response to medication, along with the degree to which activation correlates with medication-induced improvements in symptom severity, should help in identifying the neural systems that mediate the therapeutic effects of stimulants.
Discussion
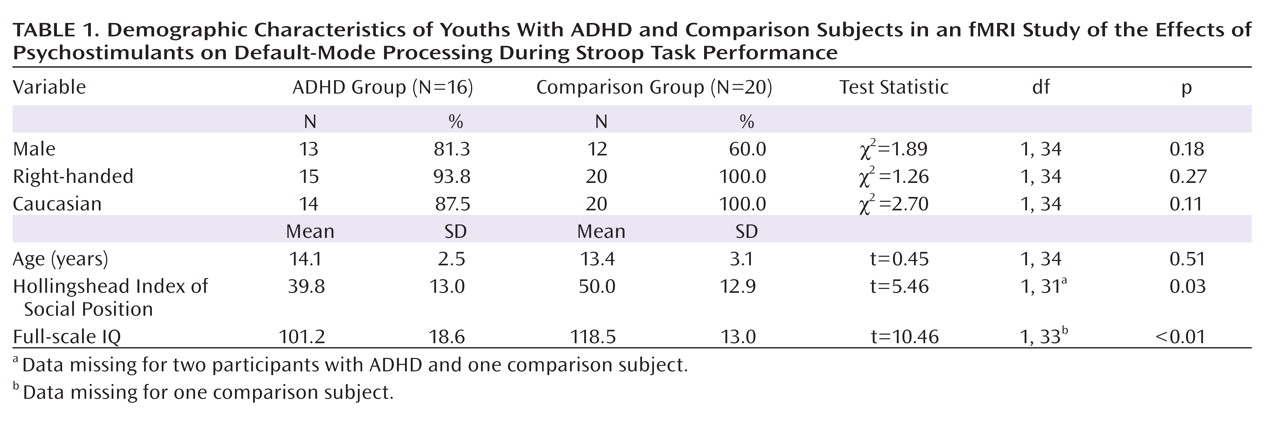
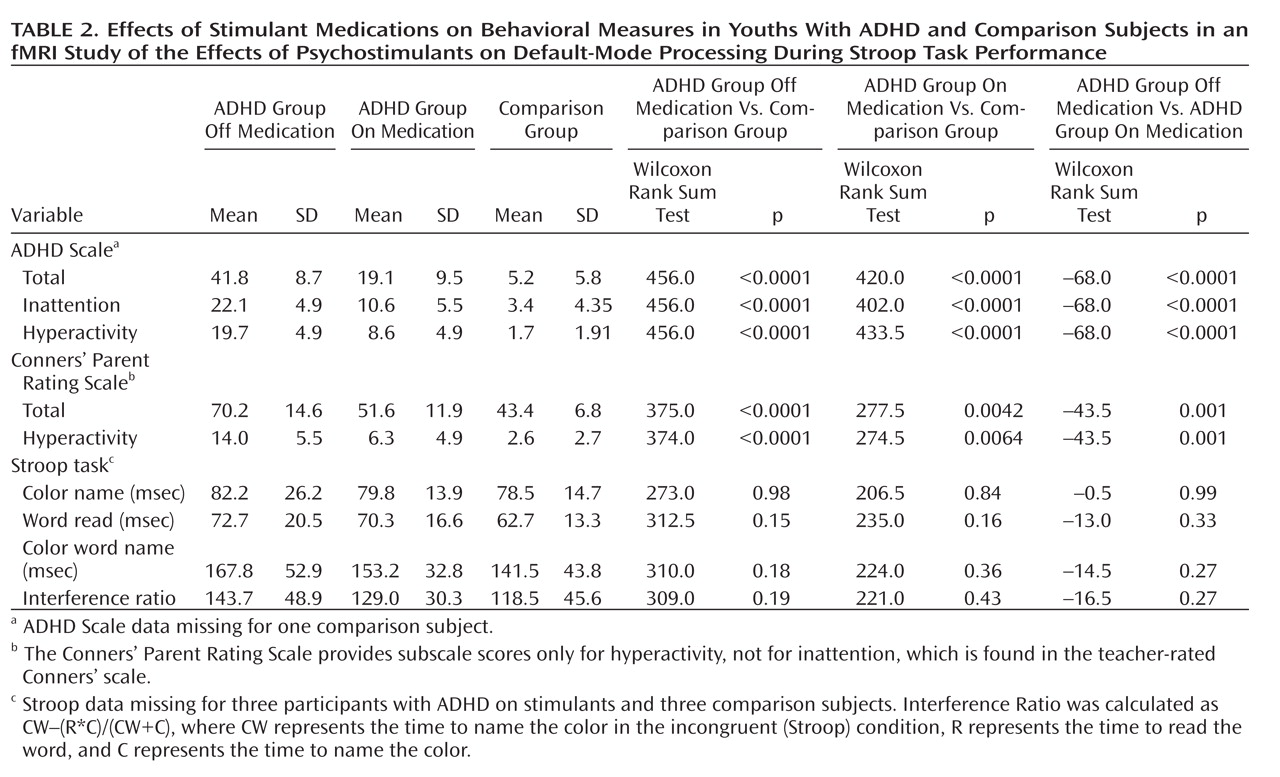
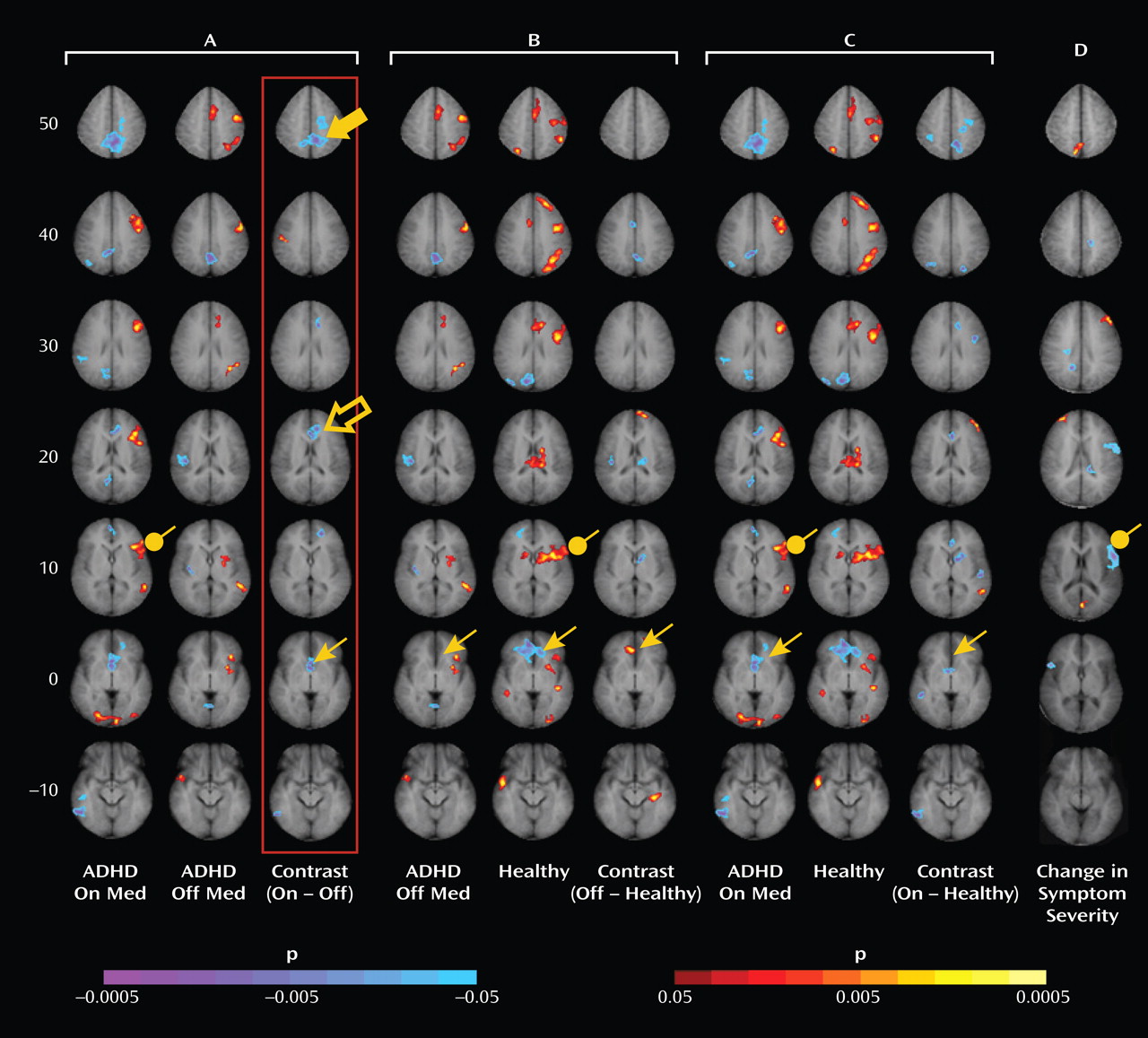
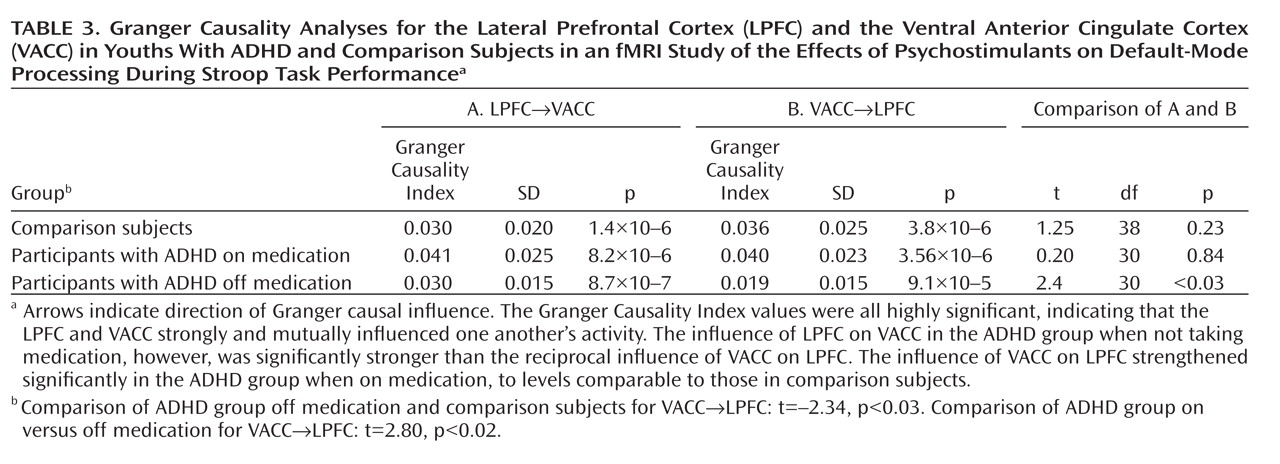
Administration of psychostimulants in youths with ADHD significantly improved the symptoms of hyperactivity and inattention. A similar but less pronounced effect was observed for measures of Stroop task performance. Stimulants also made task-related deactivations in the ventral anterior cingulate cortex and posterior cingulate cortex significantly more prominent in youths with ADHD, producing levels similar to those in healthy comparison subjects, and increased activation of the lateral prefrontal cortex to comparison group levels, although the change did not reach statistical significance. Greater activation of the lateral prefrontal cortex when off stimulants predicted a greater reduction in ADHD symptom severity when on stimulants. Causality analyses indicated that functional interactions between the ventral anterior cingulate cortex and the lateral prefrontal cortex were reduced in the ADHD group when off medication compared with interactions in comparison subjects and that these interactions in the ADHD group increased to normal levels when on stimulant medication. These findings suggest that stimulants improve symptoms in youths with ADHD by normalizing activity within a distributed network of brain regions in the anterior cingulate cortex and posterior cingulate cortex and by improving the functional interactions of that circuit with the lateral prefrontal cortex.
Numerous studies have reported deactivations in the ventral anterior cingulate cortex and posterior cingulate cortex across a wide range of tasks in both children and adults
(27,
28) . Deactivation reflects either increased activity during the less challenging control blocks of stimuli or the suppression of this activity during the more challenging active task condition. Which of these alternatives produces deactivation cannot be determined without absolute measures of brain activity in the resting condition, and our study, similar to most studies reporting deactivations, did not include these measures. Nevertheless, greater neural activity in these regions during an easier baseline condition is thought to represent a greater degree of non-task-related mental activity (termed “default-mode” activity) during the baseline condition, activity that must be suppressed during the more challenging active task for optimal performance. Indeed, the magnitude of deactivation in these regions correlates inversely with the frequency of interruptions by task-unrelated thoughts
(29,
30), which suggests that mind-wandering during the easier baseline task and its suppression during the active task produces more prominent deactivation. Deactivations are more prominent in healthy adults than in healthy children performing the Stroop task
(28), which suggests that adults may more effectively suppress default-mode activity during the more difficult task condition. The absence of deactivations in children with ADHD when not taking stimulant medication suggests either that they did not generate default-mode activity during the easier baseline condition or that they did not successfully suppress it during the attentionally more challenging active task. Greater deactivation in default-mode circuits during the administration of stimulant medications, when ADHD symptoms were improved, suggests that the most parsimonious interpretation of the absence of deactivation when not taking medication is a failure to suppress default-mode processing during performance of the more difficult active task.
The ventral anterior cingulate cortex interconnects the orbitofrontal cortex, temporal pole, amygdala, ventral striatum, and hypothalamus. It is an anatomical crossroads that contributes to the motivational processes required for goal-directed behaviors. Lesions of this area produce premature, or “impulsive,” responding in rats, impaired social valuation in macaques, and distractibility, inattention, and emotional unconcern in humans
(17) . Thus, default-mode activity in the ventral anterior cingulate cortex may reflect the motivational, affective, and rewarding properties of mind-wandering during the easier baseline task
(31) . Activity in the ventral anterior cingulate cortex, and therefore these associated subjective experiences, presumably must be suppressed to reduce distraction and improve performance during the more difficult attention-demanding task.
Previous anatomical and functional imaging studies support involvement of the default-mode circuit or related, anatomically connected brain regions in the pathogenesis of ADHD. Reduced volumes and cortical thinning in the ventral anterior cingulate cortex have been demonstrated in both cross-sectional and longitudinal imaging studies of ADHD
(32,
33) . Reduced gray matter volumes
(34) and reduced activation in proportion to symptom severity during inhibitory tasks
(3,
5,
6) have also been detected in the posterior cingulate cortex. The sizes of the lateral prefrontal cortex and anterior temporal cortices bilaterally are reduced in children with ADHD
(35), both of which are densely connected with the ventral anterior cingulate cortex
(36) . Disturbances in resting-state activity of the ventral anterior cingulate cortex and posterior cingulate cortex have been reported in adults with ADHD
(37) . Finally, methylphenidate decreases blood flow to the ventral anterior cingulate cortex, posterior cingulate cortex, and inferior parietal cortex in adults with ADHD in direct proportion to improved performance on cognitive tasks, which has been attributed to an improved efficiency of neural processing in these regions and the reduced mind-wandering induced by stimulants
(38) .
Several considerations suggest that stimulants may have produced greater suppression of default-mode activity in the ventral anterior cingulate cortex in our study by increasing activation of the lateral prefrontal cortex and strengthening the interactions of the lateral prefrontal cortex with the ventral anterior cingulate cortex. First, the lateral prefrontal cortex is known to regulate activity in multiple brain regions and neural systems
(39), and its anatomical connections with the ventral anterior cingulate cortex and posterior cingulate cortex
(36) suggest that the lateral prefrontal cortex may regulate activity in the default-mode system as well. Second, a stronger inverse coupling of activity in the lateral prefrontal cortex with default-mode activity in the ventral anterior cingulate cortex predicts improved behavioral performance on Stroop-like tasks in healthy persons
(40) . Moreover, causality analyses have provided compelling evidence that activity in the lateral prefrontal cortex plays a crucial role in controlling default-mode activity in the ventral anterior cingulate cortex across a wide range of tasks
(41) . Consistent with these prior findings, our Granger causality analyses indicated that activity in the lateral prefrontal cortex and ventral anterior cingulate cortex mutually influence one another but that the influence of the ventral anterior cingulate cortex on the lateral prefrontal cortex is significantly reduced in youths with ADHD off medication relative to comparison subjects and that it increases significantly to normal levels when youths with ADHD are on stimulants. Third, previous studies have reported that stimulant medications increase activation of the lateral prefrontal cortex in children with ADHD during performance of cognitive control tasks
(1,
11) . Consistent with this prior finding, activation of the lateral prefrontal cortex increased to normal levels in our ADHD participants when they were on stimulants. Finally, greater lateral prefrontal cortex activation in our ADHD group off medication predicted a greater reduction in symptoms following medication. Together with our finding of increased functional interactions with the ventral anterior cingulate cortex while on stimulants and the prior evidence that activity in the anterior cingulate cortex indexes mind-wandering
(29,
30), this finding suggests that greater activation of the lateral prefrontal cortex off medication may indicate a greater capacity to respond to stimulants by increasing functional interactions with the ventral anterior cingulate cortex and that this greater functional interaction is required to suppress default-mode activity and the mind-wandering that it indexes.
The findings and interpretations of this study should be considered in light of its limitations. First, our findings of differing brain activation across diagnostic groups could reflect the use of differing strategies to perform the task, rather than differences in the degree of suppression of default-mode activity. Normalization of this activity in response to stimulant medication, however, speaks against this possibility, as stimulants would be unlikely to alter task strategies. Second, subvocalization was selected as the response modality because it reduces fMRI artifacts caused by overt speech, it produces more robust Stroop interference than does a manual response, and use of a button press instead of a vocal response introduces a complex mapping of color names to finger response that fundamentally changes the nature of the task. Subvocalization, however, required measurement of task performance after the scan rather than during it, and therefore differential practice or habituation effects may have contributed to group differences in behavioral performance. Weighing against this possibility is that performance did not change with time during repeated behavioral testing in the ADHD group. Moreover, previous studies have shown similar performance and brain activation using either overt or covert verbal responses during the Stroop task
(42) . Third, including both correct and incorrect responses in our block-design fMRI analyses may have allowed the effects of error processing to contaminate activation maps. Previous event-related Stroop studies, however, have demonstrated error rates of 3% in the scanner
(43), which is consistent with error rates noted outside of the scanner in this study, and inclusion of erroneous trials at these low rates would have a negligible effect on activation maps. Fourth, differences in reading proficiency could cause group differences in brain activation, but given comparable performance across groups on the word-reading subtest of the Stroop task, this potential confounder is unlikely. Fifth, the presence of comorbid illnesses and lower IQs in the ADHD group could have contributed to our results, although statistical covariation for these factors did not change any findings. Moreover, comorbidities would not invalidate within-subject analyses comparing brain activation on and off stimulants. Sixth, our findings cannot be generalized to youths with ADHD who are medication-naive, as our study included by design only children who were known robust responders to stimulant medication. Nevertheless, our findings do generalize to the vast number of children who take and benefit from stimulant medications. Seventh, we did not rescan youths in the comparison group, which would have helped to estimate the effects of habituation across all youths in the study, but at a substantial financial cost. The counterbalanced randomization adequately controlled for habituation effects in the ADHD group, however, and permitted us to address the central scientific question of the study, which was whether stimulants alter brain functioning during a selective attention task in youths with ADHD, and not whether rescanning produces habituation during the task in healthy youths. Finally, this study did not include a placebo arm in the ADHD group, and therefore we cannot exclude the possibility that the findings may also reflect placebo effects. The ADHD youths, however, were highly robust and enduring responders to stimulant medications, and stimulant effects in general are highly robust compared to placebo in this population, which suggests that a placebo response is an unlikely cause of their symptomatic improvement and changes in brain activity.
Despite its limitations, this study is, to our knowledge, the first to demonstrate explicitly that stimulant medications improve suppression of default-mode processing during an attentional task in youths with ADHD. Our findings support the previously stated hypothesis that the failure to suppress mental processes associated with default-mode neural processing in the ventral anterior cingulate cortex and posterior cingulate cortex may contribute to the symptoms of ADHD
(37,
44) . Stimulants improved suppression of this activity in known robust responders to stimulant medication. With improvement in this suppressive activity, brain activation generally normalized and parent-reported symptoms of ADHD improved, as did objective measures of performance during attentional tasks.