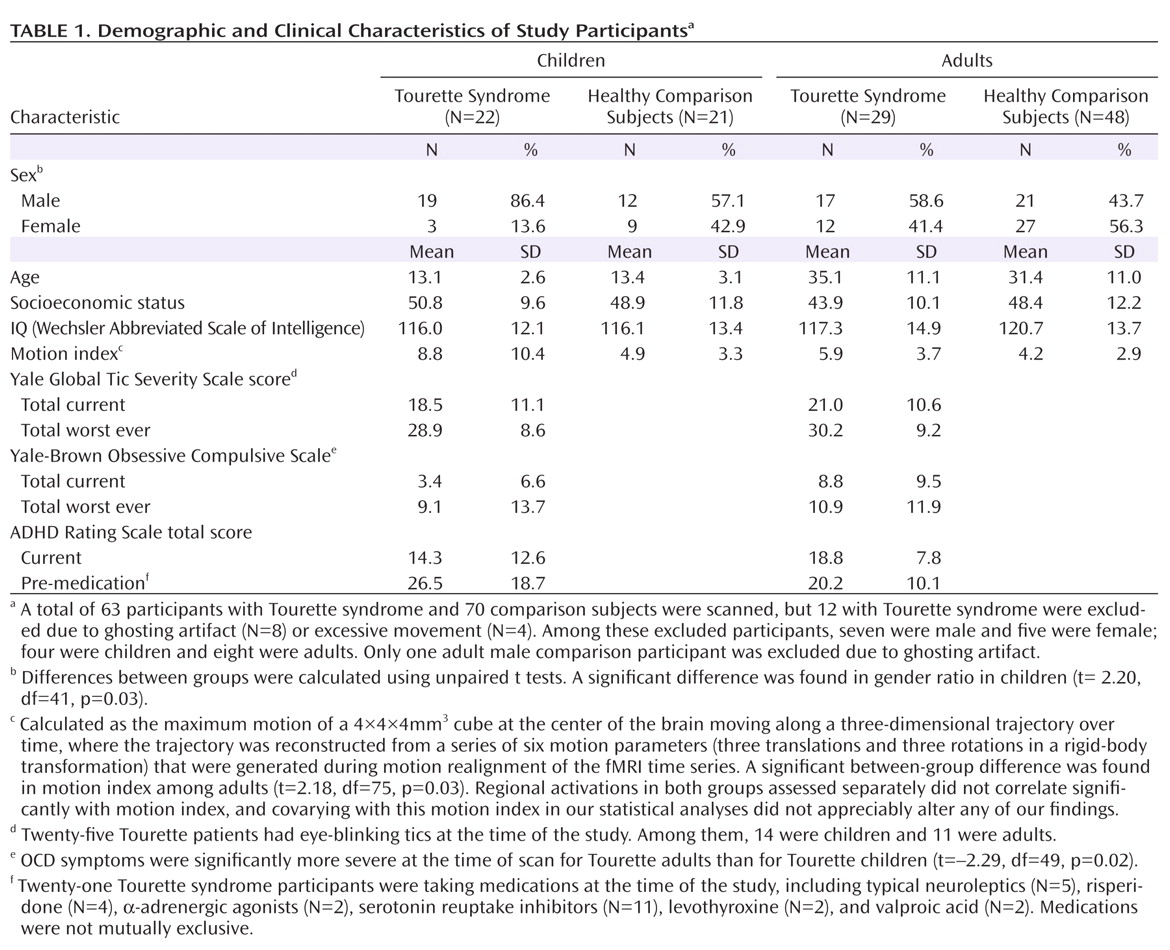
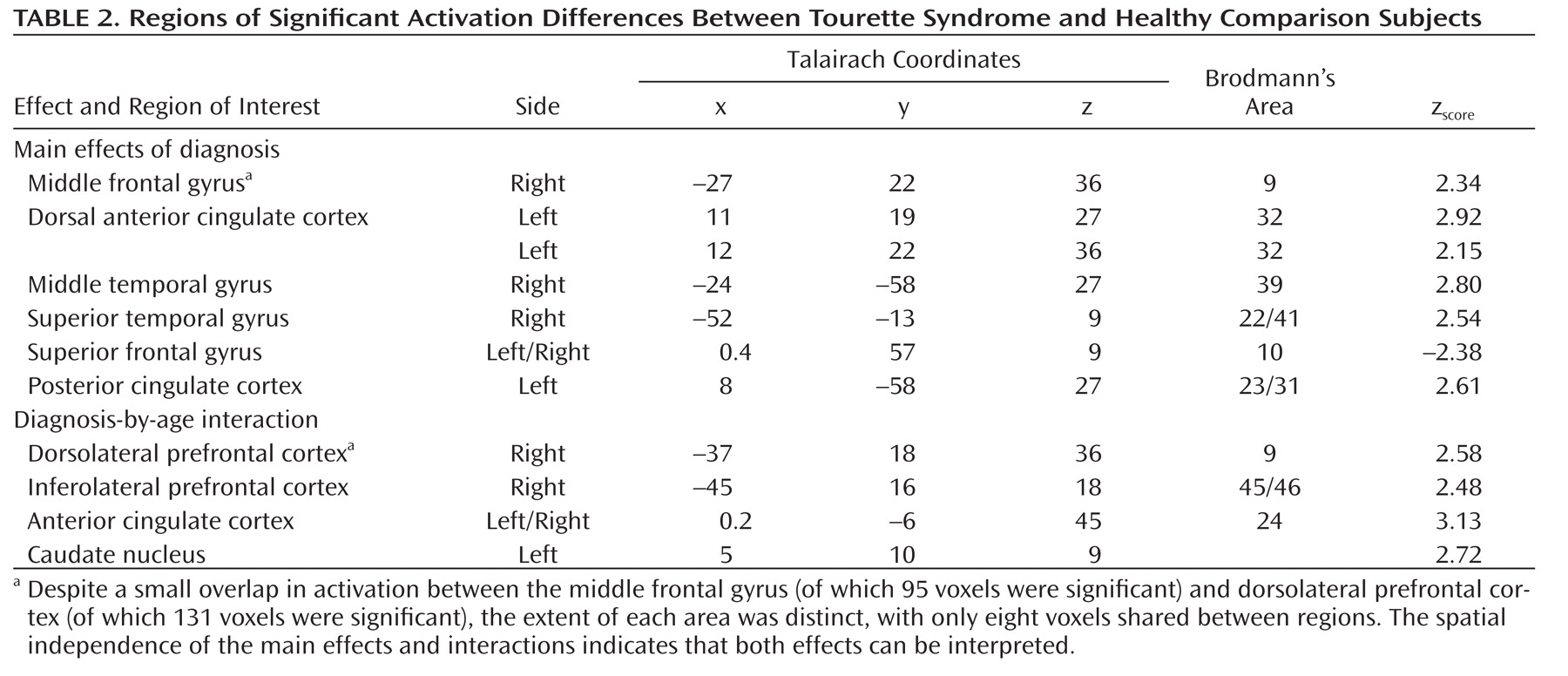
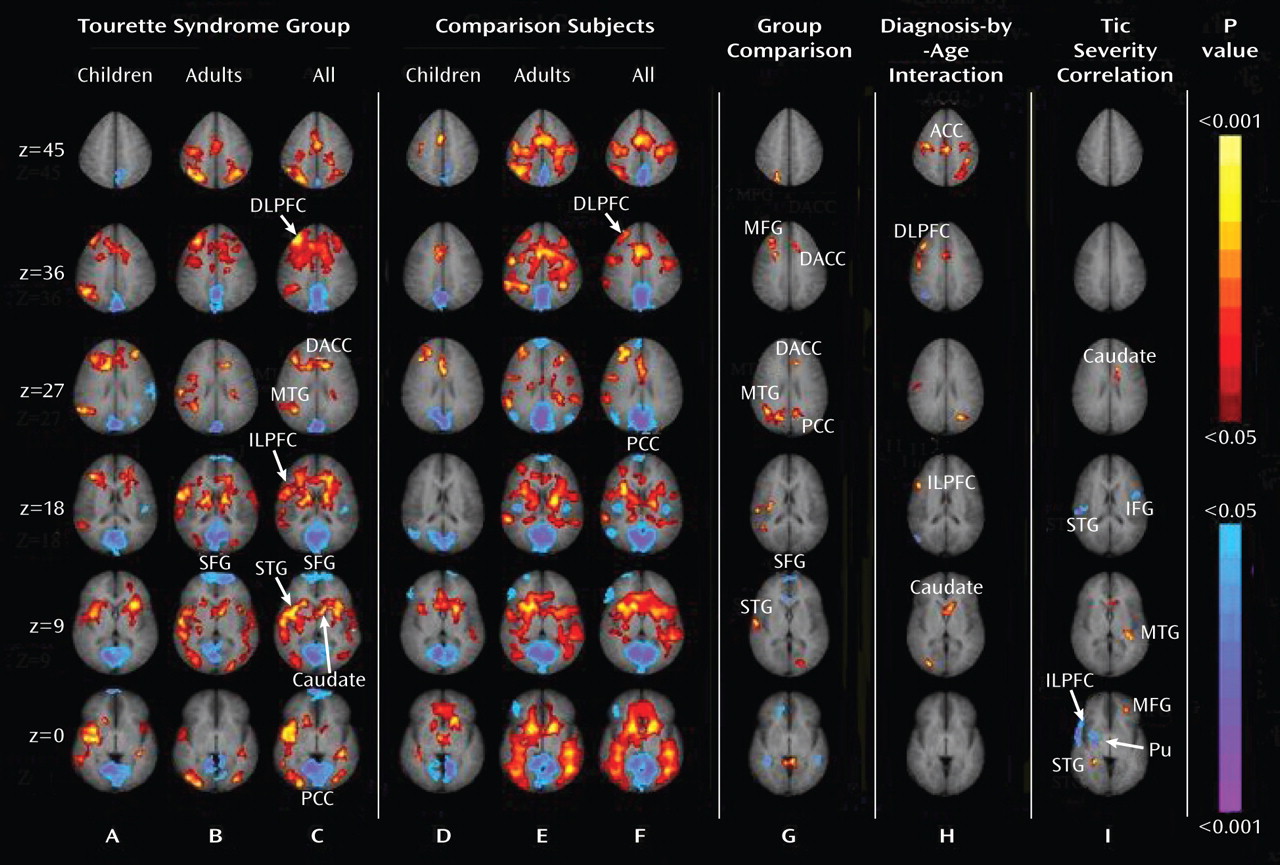
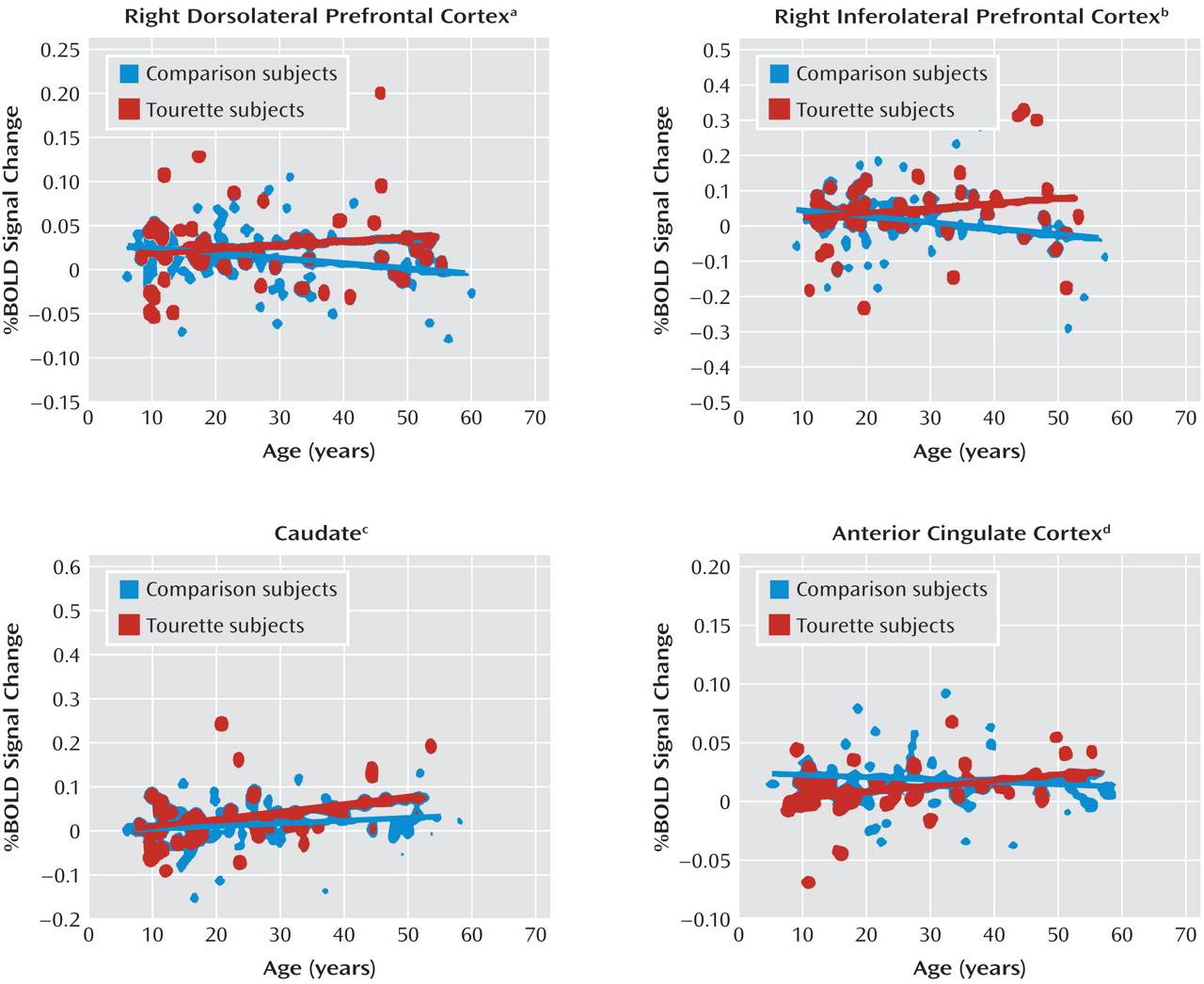
Tics are sudden, repetitive, stereotyped movements or vocal utterances that are performed in response to somatosensory or environmental cues (
1). Individuals often experience an urge to respond to these cues by performing a tic (
2). Tics can be suppressed but not indefinitely, and their temporary suppression often exacerbates the proceeding urge. The frequent need to suppress tics can be exhausting and can have a profound effect on psychosocial functioning (
1).
Prior anatomical imaging studies have documented reduced volumes of the caudate nucleus (
3) and thinning of primary sensory, primary motor, and premotor cortices in children with Tourette syndrome (
3,
4), suggesting the presence of hypoplasia throughout the motor portions of cortico-striatal-thalamo-cortical circuits. Electrophysiological and histological evidence for reduced GABA-ergic tone in these cortical and subcortical brain regions suggests the presence of reduced local inhibition of neural activity in these motor circuits (
5,
6). Prior studies of voluntary tic suppression (
7) and cortical volumes (
8) have suggested that suppression of tics robustly activates the frontal cortex, and that this frequent activation in social settings may in turn produce a compensatory, neuroplastic hypertrophy of frontal cortices that helps to regulate activity within those motor circuits, thereby reducing tic symptoms (
9). This neuroplastic hypertrophy of the frontal cortex in response to experiential need seems to be absent in adults who have persistent Tourette syndrome (
8), suggesting that a failure to generate frontal hypertrophy may contribute to the persistence of tics in the minority of persons with Tourette syndrome whose symptoms fail to remit during adolescence (
9,
10).
Although these prior studies have highlighted the importance of frontostriatal activation for the voluntary control of tic symptoms, the prior functional imaging study of tic suppression could not directly compare activation of circuits required for the suppression of tics in persons with Tourette syndrome with activation in healthy comparison subjects, since the comparison subjects by definition did not have tics to suppress. Therefore, activation of inhibitory circuits in persons with Tourette syndrome has been compared instead with activation in healthy comparison subjects during the performance of cognitive tasks that require the inhibition of prepotent responses. One study, for example, compared the brain activity of children and adults with and without Tourette syndrome during performance of the Stroop Word-Color Interference Task (
11), which requires the inhibition of an automatic response tendency (reading) in favor of a less automatic response (color naming). The correlations of activation with age within frontostriatal circuits were greater in the Tourette group than in comparison subjects, suggesting the presence of altered maturation of frontostriatal circuits. Greater frontostriatal activity in the adults with Tourette syndrome was interpreted as helping them to maintain normal levels of task performance in the presence of a diminished inhibitory reserve in these circuits (
8).
We sought to develop a functional imaging task that was similar to voluntary tic suppression in the sense that it would require participants to suppress a semi-involuntary behavior but would also permit performance by healthy participants, thereby allowing a direct comparison of activity in frontostriatal circuits across Tourette syndrome and comparison groups. One semi-involuntary behavior that is phenomenologically similar to tics is eye blinking. Blinks occur at a similar frequency and with similar forcefulness as most tics, and they can be voluntarily suppressed temporarily although not indefinitely. Difficulty in regulating the frequency and forcefulness of eye blinking is one of the earliest-developing tic symptoms (
2); moreover, individuals with Tourette syndrome report that the somatosensory tension experienced during the sustained inhibition of eye blinking is similar to the premonitory urge experienced just prior to performing a tic. Functional imaging studies have shown that frontostriatal circuits are activated in healthy individuals during the inhibition of eye blinks (
13), thus making the suppression of eye blinks an ideal candidate task for comparing frontostriatal activity in suppressing semi-involuntary behaviors across the Tourette and comparison participants. This comparison should allow determination of whether the functioning of frontostriatal circuits during the inhibition of a normal, semi-involuntary behavior is aberrant, therefore suggesting the presence of a generalized deficiency in inhibitory systems in Tourette syndrome, or whether aberrant inhibition represents a domain-specific deficiency in the inhibition of tics alone.
We therefore compared neural activity in individuals with Tourette syndrome and healthy comparison subjects as they inhibited eye blinking. Given the previously reported disturbances in frontostriatal circuits of adults with Tourette syndrome during performance of the Stroop task (
11), we expected to detect a similar pattern of increased activity of frontal cortices in response to inhibitory demands in adults with persistent symptoms (
8,
11), suggesting that they increase frontostriatal activity to compensate for reduced inhibitory reserve within frontal cortices.
Discussion
Excessively frequent and forceful eye blinking is most commonly the first symptom to manifest in children with Tourette syndrome (
30). Eye blinks are semi-involuntary behaviors that have a similar duration, frequency, and forcefulness to tics themselves, and persons with Tourette syndrome report that the subjective experience while inhibiting eye blinks is similar to their subjective experience while inhibiting tics. The temporal dynamics of tics and eye blinks are similar (
31), and the frequencies of both are believed to be under the neuromodulatory control of dopamine (
32). Thus, the inhibition of eye blinks provides an ideal task to study neural circuits that provide inhibitory control over semi-involuntary movements across Tourette and healthy participants.
Numerous imaging studies have shown that the frontal cortex plays a central role in the development of inhibitory motor control (
33,
34) in both children (
35) and adults (
36). Other imaging studies have suggested that frontal control of tic behaviors is impaired in persons with Tourette syndrome, particularly adults who have persistent symptoms. Persons with Tourette syndrome, for example, have abnormalities in the structure and function of their prefrontal cortices (
7,
8). Whereas children with Tourette have enlarged frontal cortices, adults have reduced frontal volumes (
3). In addition, prefrontal volumes in both children and adults with Tourette syndrome correlate inversely with tic severity (
3), suggesting that enlargement of frontal cortices in children may represent a plastic hypertrophy in response to their frequent need to suppress tics, which is known to activate the frontal cortex robustly (
7). Plastic hypertrophy may thereby enhance inhibitory control over tics. Conversely, smaller prefrontal volumes in Tourette adults are thought to represent a failure of this plastic hypertrophy (
8), limiting inhibitory reserve and requiring an increased magnitude and spatial extent of frontal activation to maintain a comparable level of performance on tasks requiring inhibitory control (
11).
Consistent with this model of impaired inhibitory control in adults who have persistent symptoms, one prior study reported greater age-related increases in frontal activation during an inhibitory control task in persons with Tourette syndrome relative to that seen in healthy comparison subjects (
11). These findings were interpreted as a functional response in Tourette adults that likely compensated for their reduced frontal and caudate volumes (
11). We detected a similar age-by-diagnosis interaction in the right dorsolateral prefrontal cortex and right inferolateral prefrontal cortex (Figure 1, column H) that was driven by greater activation with advancing age in the Tourette group than in comparison subjects (Figure 2). These two studies included most of the same participants, demonstrating the within-subject reproducibility of this set of findings. These consistent findings across studies suggest that the exaggerated deployment of inhibitory control in Tourette adults likely extends beyond the more cognitive domain of the Stroop task and into the more purely motor domain of motoric inhibition.
Although the bulk of evidence together suggests that the greater activation of frontal cortices during the Stroop and eye blink tasks represents an attempt to compensate for the reduced plasticity of the prefrontal cortex in persons with Tourette syndrome, other interpretations can also account for greater activation of frontostriatal pathways in Tourette adults. Imaging, postmortem, and electrophysiological studies, for example, suggest the presence of hypoplasia and reduced GABA-ergic tone throughout motor portions of cortico-striatal-thalamo-cortical circuits in persons with Tourette syndrome (
5,
6). Reduced GABA-ergic tone would increase the excitability of the motor pathways that presumably drive the generation of both tic symptoms and eye blinks. Greater excitability in these motor pathways could in turn require greater activation of frontostriatal pathways to suppress those behaviors. This explanation, however, would not readily account for the increased activation of frontostriatal pathways detected in Tourette syndrome adults during performance of a cognitive control task (
11).
Our findings of increased activation of the dorsal anterior cingulate cortex and right anterior temporal cortex in the Tourette group relative to comparison subjects during eye blink inhibition is consistent with prior findings of increased activation of these areas during tic suppression in persons with Tourette syndrome (
7). The dorsal portion of the anterior cingulate cortex is believed to subserve attention and monitoring functions during the performance of goal-directed tasks (
37), and the right anterior temporal cortex subserves attention and the processing of visuospatial and somatosensory information (
38). Thus, increased activity in these areas in the Tourette group may represent a greater reallocation of attentional resources than in comparison subjects during the suppression of eye blinks, perhaps reflecting a more generalized difficulty with motor inhibition across all ages in the Tourette group.
In addition to activating frontostriatal pathways more than comparison subjects, Tourette subjects deactivated the superior frontal gyrus more than comparison subjects during the suppression of eye blinks. The superior frontal gyrus is thought to support the processing of self-referential information and metacognitive representations of the actions of self and others (
39). More prominent deactivation of the superior frontal gyrus in the Tourette group likely reflects a greater suppression of self-directed thought during the suppression of eye blinks, suggesting that this task is more demanding in persons with Tourette syndrome than in comparison subjects. In contrast, the Tourette group failed to deactivate the posterior cingulate cortex to the extent that healthy comparison subjects activated it. The posterior cingulate is thought to support the monitoring and recall of somatic and other sensory information (
37). Failure to turn off this region sufficiently during eye blink suppression in the Tourette syndrome group may represent a disturbance in the ability to suppress somatic sensations associated with the urge to blink when persons with Tourette syndrome willfully suppress their eye blinks.
We detected inverse correlations between the current severity of tic symptoms and the magnitude of activation of the right inferolateral prefrontal cortex, right superior temporal gyrus, and right putamen during the inhibition of blinks, and we detected a positive correlation in the middle frontal gyrus and caudate. These correlations are consistent with those reported previously during tic suppression (
7). The inverse correlations indicate that individuals with more severe tics, and by extension those who had greater difficulty suppressing tics, activated these regions less than did those who had less severe symptoms. The correlations provide further support for our claim that activation of frontostriatal circuits provides inhibitory control over semi-involuntary movements, whether these are tics or eye blinks. Greater functional disturbances in frontostriatal systems may therefore contribute to more severe symptoms.
These findings must be interpreted in light of several limitations of the study. First, variability in task compliance could produce differences in neural activity. To minimize this variability, a member of the study team directly observed each participant during the scan to confirm their success in suppressing blinks. Second, the presence of co-occurring illnesses and use of medications could have influenced our results. Weighing against this possibility, however, is that covarying for medication use and co-occurring illnesses had no discernible effects on our findings. Third, we were unable to quantify the number of eye blinks during the freely blinking control task because bursts of blinks occurred immediately following suppression blocks, rendering their enumeration impossible (
31). The Tourette and comparison groups therefore may have differed in their frequency of eye blinking during the resting condition, thereby influencing estimates of task-related change in MRI signal. Quantitative studies of blink rates, however, generally have not reported abnormal blink rates in persons with Tourette syndrome (
40). Finally, we did not include Tourette adults with remitted symptoms, and therefore we could not assess whether exaggerated frontostriatal activation generalizes to all adults with a lifetime history of Tourette syndrome or only to those whose symptoms persist. Despite these potential limitations, our study has important implications for understanding the developmental trajectory of brain functioning in Tourette syndrome. In particular, it provides new support for the hypothesis that disturbances in the maturation of frontostriatal systems that mediate inhibitory control can contribute to the development, persistence, and severity of tic symptoms.