A response to a single antidepressant medication, classically measured as an attenuation of 50% or more in the intensity of depressive symptoms, is generally obtained in about 50%–75% of patients with a first trial (
1–
3). It is recognized, however, that leaving patients with residual symptoms predisposes them to a full relapse of depression within a much shorter time than if remission were achieved (
4,
5). Remission rates, in contrast, are generally around 30% with a single agent (
1–
3). This is a disappointing result indicating that additional treatment measures must be taken in about two-thirds of patients after using an antidepressant medication at an adequate dose for a sufficient time. The current standard of care would be drug substitution, carried out with or without an elimination period, depending on the choice of medications. Given that an antidepressant drug trial should last at least 6 weeks, two consecutive attempts using different medications requires about 3 months. Considering that about half of all patients stop their antidepressant drug within this time frame (
6,
7), it is clear that initial treatment steps are critical to achieving the goal of remission.
Another common approach is to maintain the first drug and add another agent, with or without intrinsic antidepressant action, to obtain remission. It is not proven, however, that drug addition is more effective than drug substitution (
8–
12). Nevertheless, the strategy of adding a second medication to an ongoing antidepressant drug regimen requires completion of a first trial, thereby still delaying response or remission in most patients.
Some researchers have conducted studies using two medications from treatment initiation in an attempt to obtain either a more rapid onset of therapeutic action or greater efficacy (see reference
13 for a review). In a 6-week randomized, double-blind trial (
14), improvement on the Montgomery-Åsberg Depression Rating Scale was 10 points greater in patients receiving combination therapy with mirtazapine and the selective serotonin reuptake inhibitor (SSRI) paroxetine than in patients treated with either drug alone. Moreover, after the addition of the other drug in monotherapy patients who did not respond, the number of those who remitted doubled within a 2-week period. These results suggest that antidepressant drug combination from treatment initiation, in both drug-naive and treatment-resistant patients, may increase remission rates.
The primary objective of this study was to support the superiority of combination treatment with two antidepressant medications from treatment initiation compared with monotherapy. A second objective was to determine whether two antidepressant drugs other than SSRIs—namely, venlafaxine (a serotonin and norepinephrine reuptake inhibitor) and bupropion (a catecholamine releaser) (
15,
16)—could synergize with mirtazapine to produce an antidepressant effect superior to that of fluoxetine alone. A third objective was to determine whether a single drug was sufficient to maintain response in patients who achieved a marked response with combination therapy by discontinuing one medication on a double-blind basis after 6 weeks of treatment.
Method
This study was carried out at three sites. It was first implemented at the University of Florida, where the first 65 patients were studied. The principal investigator (P.B.) and the study coordinator (C.H.) then relocated to the University of Ottawa Institute of Mental Health Research (IMHR) in Ontario. The study was then completed at the IMHR and at a satellite clinic at the Centre Hospitalier Pierre Janet, in Hull, Canada.
Patients
All patients were recruited from newspaper advertisements approved by the research ethics boards at the three sites, from the Mood Disorders Clinic of the Royal Ottawa Hospital, and from consultation requests from community physicians. Selection for the study was based on a psychiatric examination and a physical examination followed by a full blood workup including basic hematology and biochemistry, thyroid function tests, urinalysis, serum human chorionic gonadotropin levels for nonmenopausal women, and a urine drug screen. Patients were then assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (
17). For inclusion in the study, patients had to meet DSM-IV criteria for major depressive disorder (
18) as a primary diagnosis and had to have a score of at least 18 on the first 17 items of the Hamilton Depression Rating Scale (HAM-D;
19). Exclusion criteria included nonresponse to fluoxetine in the index episode, an unstable medical condition, a history of a seizure disorder, abnormal and clinically significant values in the blood workup, and a history of hypomania or mania. After screening, 105 patients gave written informed consent on a form describing the study. Psychotropic drugs were discontinued and deemed to be eliminated after five half-lives prior to randomization. Patients were then entered into the study if their HAM-D score was still at least 18, unless their score had decreased more than 20% between screening and baseline. Clonazepam (up to 1 mg/day), zopiclone (7.5 mg at bedtime), and zolpidem (10 mg at bedtime) were allowed to help manage anxiety and insomnia. There was no financial compensation for participants, but all tests, examinations, follow-up visits, and study medications were provided free of charge.
Study Design
Patients were randomly assigned, with a provision for gender, to one of four groups: fluoxetine monotherapy or combination therapy with mirtazapine plus fluoxetine, venlafaxine, or bupropion. The brand formulations of fluoxetine, mirtazapine, and extended-release venlafaxine were used throughout. The bupropion capsules were prepared from mixing 150 mg of the original bupropion powder with methylcellulose to obtain a slow-release preparation. This daily regimen of bupropion was shown in a randomized controlled trial (
20) to be superior to placebo and as effective as 300 mg/day. Treatment regimens were kept constant for the first 6 weeks, with the exception of the venlafaxine arm, in which patients received 75 mg/day during week 1, 150 mg/day during week 2, and 225 mg/day during the last 4 weeks in order to reliably block norepinephrine reuptake (
15,
21). Patients were instructed to take capsules morning and evening from different bottles. All patients were instructed to take one capsule from one bottle approximately 30 to 45 minutes before going to bed throughout the trial; for patients assigned to combination treatment, this capsule contained mirtazapine, and for those in the fluoxetine monotherapy arm, it contained a matching placebo. During week 1, all patients were instructed to take one capsule in the morning from another bottle; for patients in the fluoxetine monotherapy group and the fluoxetine combination group, this bottle contained fluoxetine, whereas for the other two combination groups, it contained bupropion or venlafaxine. During week 2, patients were instructed to take one capsule in the morning from each of two bottles: in the two fluoxetine arms, bottle A contained fluoxetine and bottle B a placebo; in the venlafaxine arm, both bottles contained 75-mg capsules of venlafaxine; and in the bupropion arm, bottle A contained 150-mg capsules of bupropion and bottle B a placebo. From week 3 on, the contents of the bottles were the same as for week 2 except in the venlafaxine arm: bottle A contained 150-mg capsules of venlafaxine and bottle B contained 75-mg capsules of venlafaxine. All medications were supplied in identical capsules.
In the discontinuation and prolongation phase, there was no modification of capsule content in the fluoxetine monotherapy arm. In the mirtazapine-fluoxetine combination arm, the evening mirtazapine capsule was abruptly switched to a placebo. In the mirtazapine-venlafaxine combination arm, the venlafaxine was decreased to 150 mg/day for 1 week, then to 75 mg/day for 1 week, and then was discontinued. In the mirtazapine-bupropion arm, the morning capsule of bupropion was abruptly switched to a placebo. Consequently, patients were on either fluoxetine or mirtazapine for the 6-month prolongation.
Ratings
Patients were assessed at day 4, 7, 10, 14, 21, 28, 35, and 42 using the HAM-D, the Montgomery-Åsberg Depression Rating Scale (MADRS;
22), and the Clinical Global Impressions (CGI;
23) severity scale and, for all visits after day 1, the CGI improvement scale. No more than a 1-day variance was allowed for the first four visits, 3 days for the subsequent weekly visits, and 1 week for the monthly visits. The primary outcome measure was determined a priori to be the change in HAM-D score.
Remission, Response, and Relapse Criteria
Treatment remission was defined as a sustained score of 7 or less on the HAM-D (
1,
25). Treatment response was taken as a sustained improvement of 50% or more in HAM-D score. To enter the prolongation phase, patients had to reach a score of 12 or less on the MADRS, which is considered a less stringent remission criterion. In the prolongation, the patients were considered to be relapsing if they met DSM-IV criteria for major depressive disorder and if their MADRS scores were greater than 12 for two consecutive visits, either 2 or 4 weeks apart depending on the severity of the symptoms. The blind was not broken in case of relapse.
Determination of Plasma Levels of Mirtazapine
Blood samples were collected at day 28 for determination of plasma levels of mirtazapine. Blood analysis was carried out by high-performance liquid chromatography with fluorescence detection by Organon Pharmaceuticals in Oss, the Netherlands. Samples were stored at –70°C until shipped.
Monitoring of Physiological Parameters and Adverse Events
Sitting blood pressure, pulse, and weight were measured at each visit. Patients were questioned at each visit for the occurrence of adverse events.
Statistical Analyses
Ratings were carried out on the basis of all patients who underwent randomization and received at least one dose of study medication (all patients treated). The last-observation-carried-forward method was applied throughout. The scores were analyzed by means of two-way analysis of variance for the HAM-D, the MADRS, and the CGI subscales followed by post hoc tests to assess statistical differences between groups. Covariance analyses were not used since the baseline scores between the groups were nearly identical. Comparisons of the numbers of patients who responded and remitted were made with chi-square tests. Statistical significance was assessed using an alpha of 0.05 (two-tailed).
Results
Demographic and Clinical Characteristics
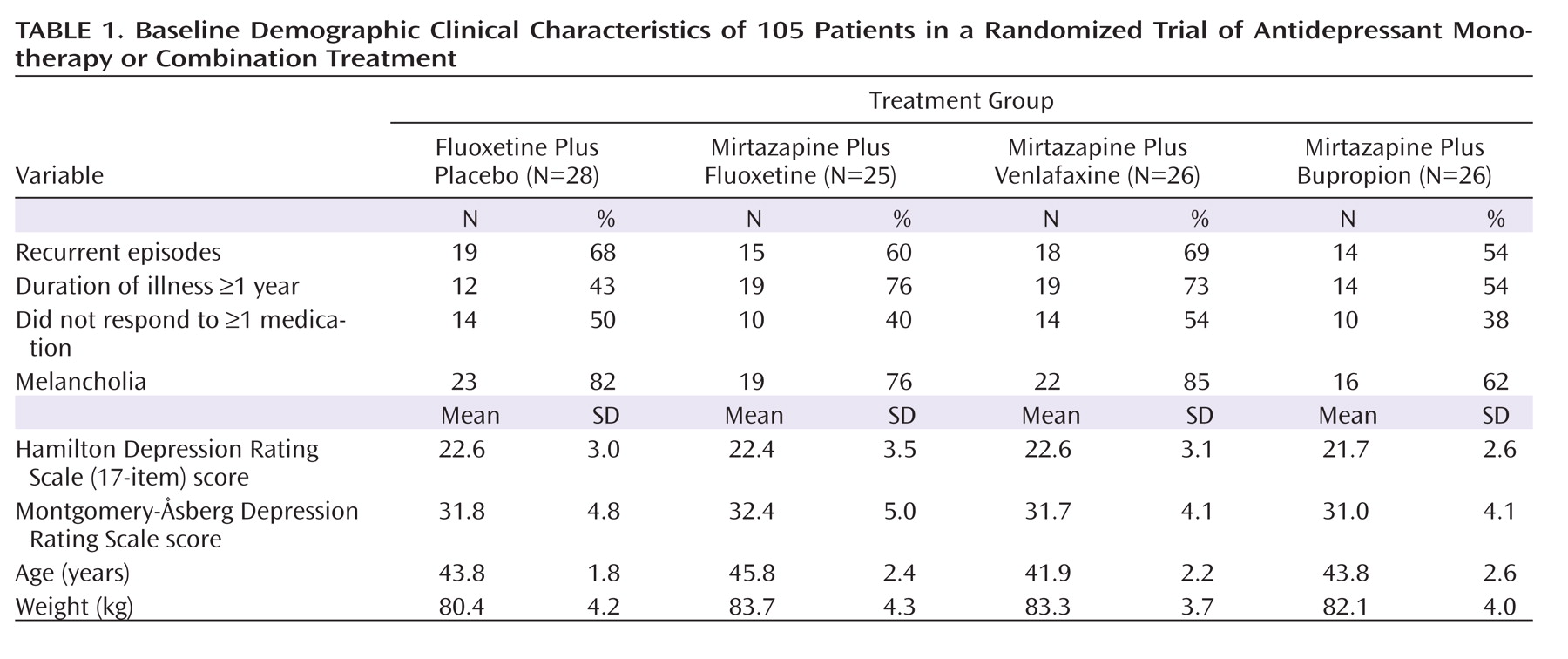
The number of patients with recurrent major depression, the duration of the index episode, and the proportion of patients who failed at least one adequate trial of an antidepressant were comparable in all groups. The patient population on average presented with moderate to severe depression, with a mean HAM-D score of about 23 and a mean MADRS score of about 32; about two-thirds of the patients met DSM-IV criteria for melancholia (
Table 1).
Plasma Levels of Mirtazapine
The plasma levels of mirtazapine at day 28 in the combination groups were consistent with those previously reported with mirtazapine monotherapy at 30 mg/day (
26) and were not significantly different from each other (fluoxetine [N=21], mean=43 ng/ml, SD=18; bupropion [N=21], mean=35 ng/ml, SD=14; venlafaxine [N=24], mean=37 ng/ml, SD=20).
Tolerability and Compliance
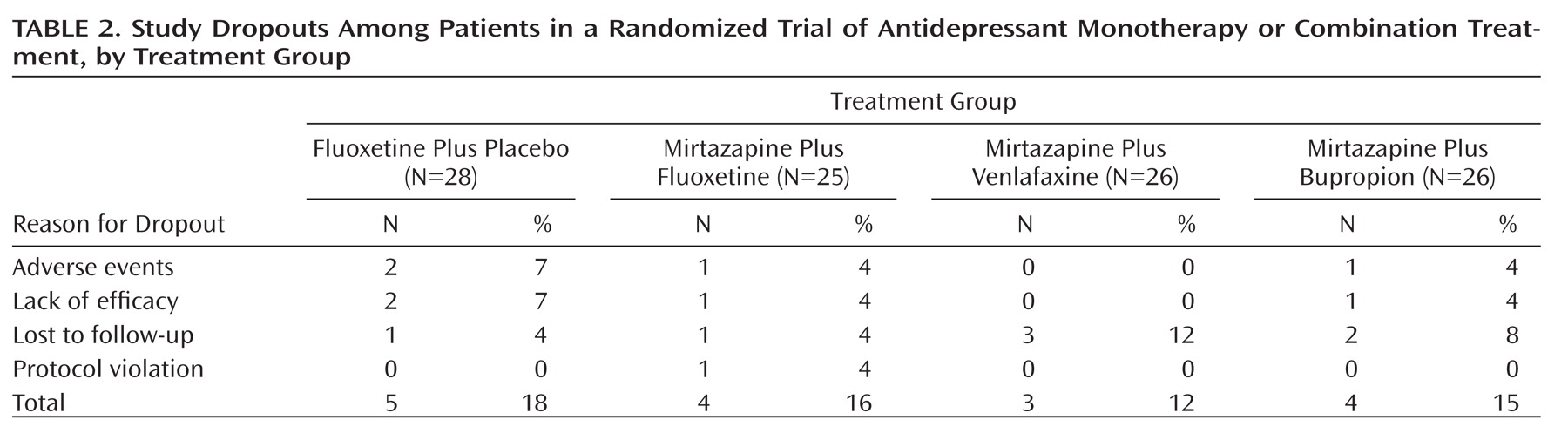
Sixteen patients (15%) dropped out of the study during the first 6 weeks (
Table 2). Of the 66 patients who consented to enter the 6-month prolongation, about one-third dropped out. Only one patient was dropped from the prolongation because of noncompliance. Otherwise, compliance was well within 80%–120% based on pill counts.
Clinical Efficacy During the First 6 Weeks
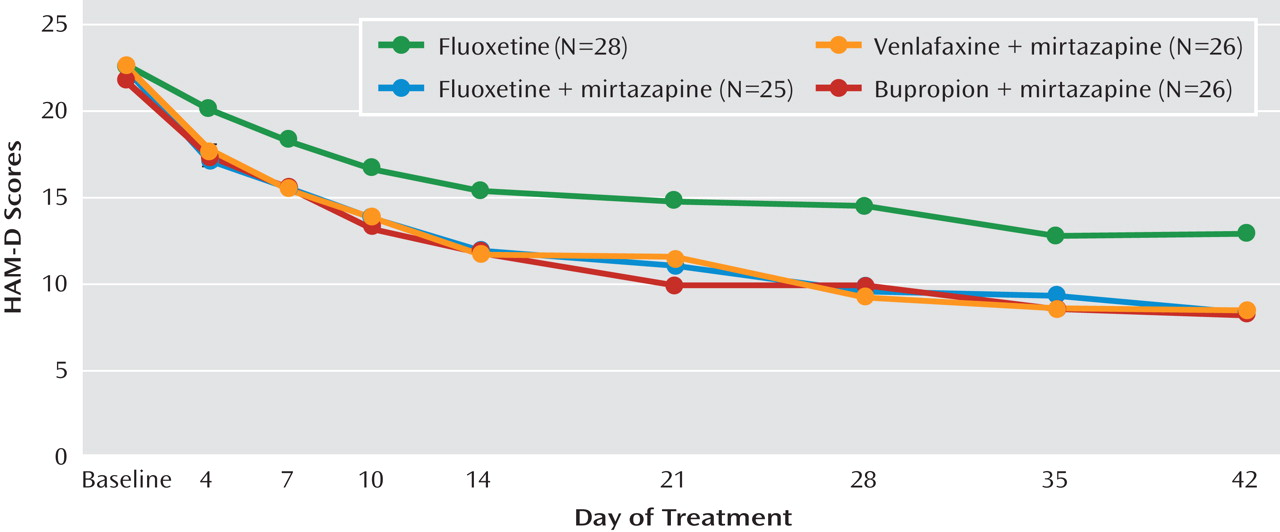
There was a statistically significant difference among the different time points on the HAM-D (F=144.3, df=8, 101, p<0.001). Post hoc analyses showed that HAM-D scores were significantly different baseline from day 7 onward in the fluoxetine monotherapy arm and from day 4 onward in the three combination groups. HAM-D scores were statistically significantly different among the four treatment groups (F=3.87, df=3, 101, p=0.011). Post hoc analyses showed that such differences were significant when comparing the fluoxetine monotherapy group with the three combination groups. In comparisons of groups within each time point, the bupropion combination group separated from the fluoxetine monotherapy group starting at day 21, whereas the fluoxetine and venlafaxine combination groups separated at day 28, with the exception of the fluoxetine combination group at day 35. At day 42, the mean difference between the fluoxetine monotherapy group and the combination groups ranged from 4.5 to 4.8 points (
Figure 1). There was no significant group-by-time interaction.
The number of patients who achieved a response to treatment was not statistically different among the four groups (fluoxetine monotherapy, 54%; mirtazapine plus fluoxetine, 68%; mirtazapine plus bupropion, 65%; mirtazapine plus venlafaxine, 73%). The mean time to sustained response was 22 days for patients receiving fluoxetine monotherapy and those receiving mirtazapine plus venlafaxine, 15 days for those receiving mirtazapine plus fluoxetine, and 14 days for those receiving mirtazapine plus bupropion. These values were not statistically different.
The proportion of patients achieving a sustained remission was statistically different between fluoxetine monotherapy (25%) and the fluoxetine combination (52%) and the venlafaxine combination (58%), but not the bupropion combination (46%). The mean times to remission were nearly identical in all groups, at 23 or 24 days.
The difference in MADRS scores between fluoxetine monotherapy and the combination therapies at day 42 were 4.9, 4.6, and 5.8 points for the fluoxetine, bupropion, and venlafaxine combinations, respectively, although the differences did not reach statistical significance (F=2.22, df=3, 101, p=0.09). Similarly, there were no significant differences between treatment groups on the CGI severity and improvement scores (F=1.48, df=3, 100, p=0.22, and F=2.32, df=3, 98, p=0.08, respectively). However, the numbers of patients who had a score of 1 on the CGI severity scale (not ill at all) were 11, 10, and 15 in the fluoxetine, bupropion, and venlafaxine combination groups, respectively, compared with seven in the fluoxetine monotherapy group.
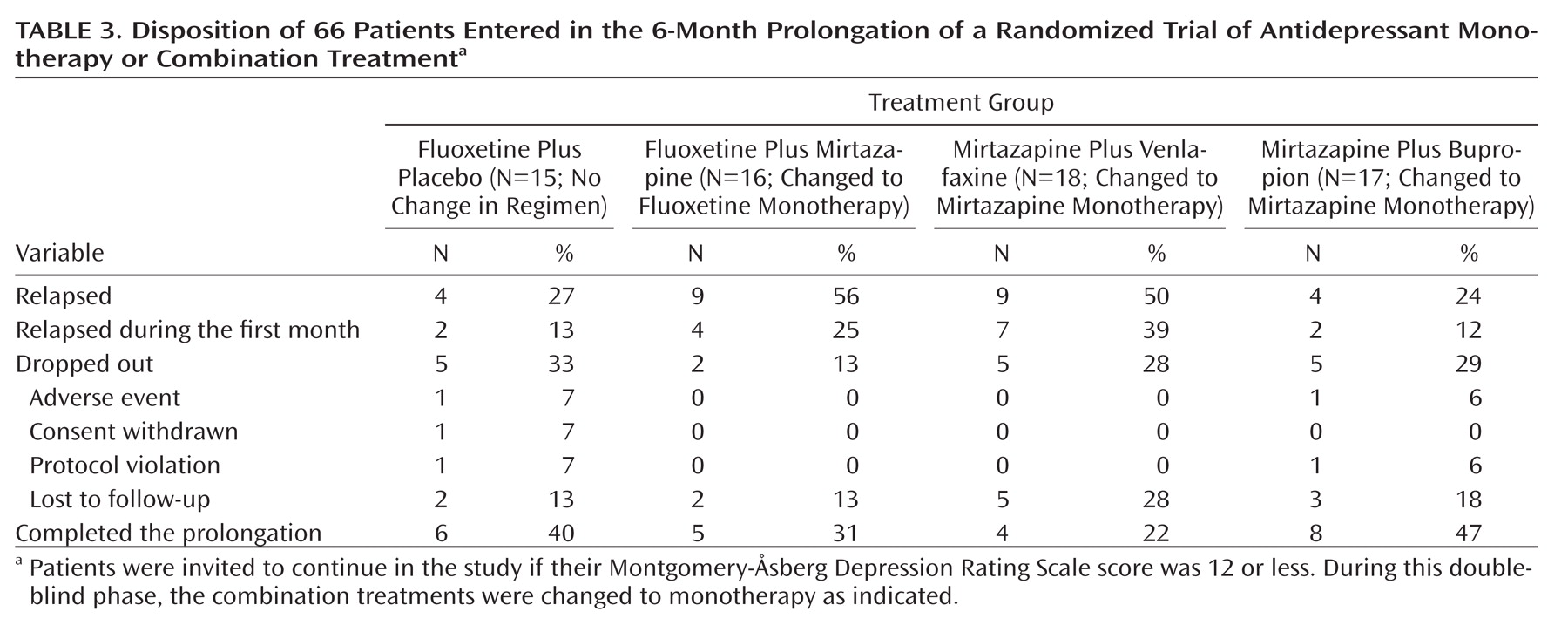
In the prolongation phase, about half the patients who initially received combination treatment with mirtazapine and fluoxetine or venlafaxine relapsed, compared with about a quarter of those who initially received fluoxetine monotherapy or combination treatment with mirtazapine and bupropion (
Table 3). About half of these relapses occurred within the first month of the prolongation. Nearly all patients promptly regained their prior improvement in symptoms when a combination treatment was restored. Only about a third of all patients who entered the 6-month prolongation completed this phase in remission, with no significant difference between those receiving fluoxetine and those receiving mirtazapine (Table 3).
Physiological Parameters and Adverse Events
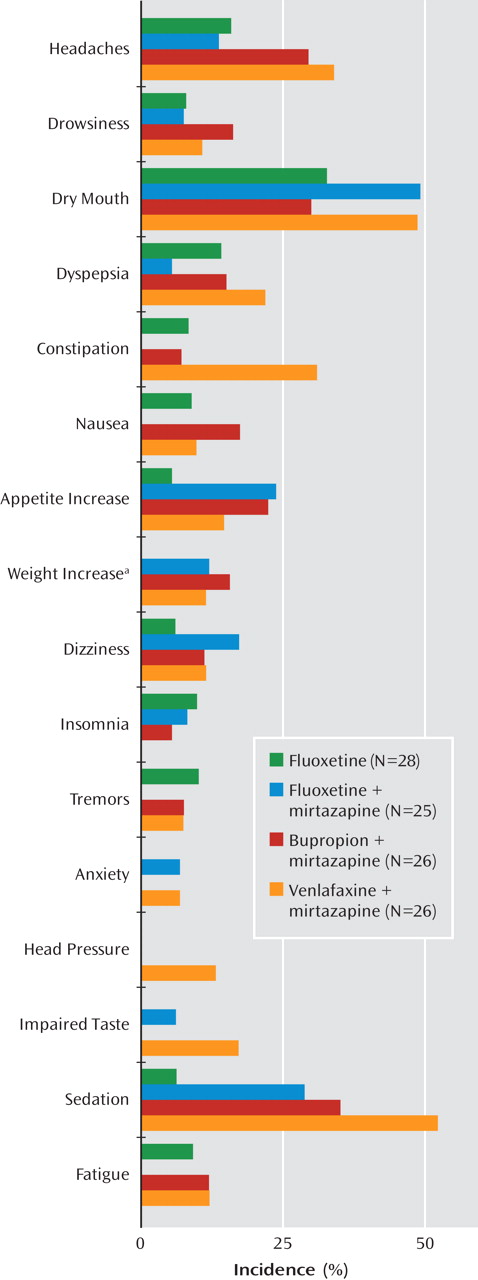
There were no significant changes in sitting systolic blood pressure or pulse (data not shown). There was no significant weight change in the fluoxetine monotherapy group at day 42 compared with baseline (mean=+0.1 kg, SD=1.5, N=25), but patients in the combination groups had significant increases (fluoxetine, mean=3.1 kg, SD=2.5, N=25; bupropion, mean=2.7 kg, SD=2.4, N=22; venlafaxine, mean=2.2 kg, SD=2.5, N=24; F=9.1, df=3, 92, p<0.001; all values were significantly different from fluoxetine monotherapy). In the 6-month prolongation, there was no significant weight change in either the fluoxetine (mean=–0.7, SD=1.9, N=10) or the mirtazapine (mean=+0.2, SD=3.5, N=12) group. The incidence of adverse events reported in at least 5% of patients is shown in
Figure 2. Five patients in the fluoxetine monotherapy group took anxiolytics/sedatives, compared with four, four, and two in the fluoxetine, bupropion, and venlafaxine combination groups, respectively.
There were no suicide attempts in any of the 105 patients. Overall, there was a highly significant decrease in suicide subscale scores—item 3 on the HAM-D and item 10 on the MADRS (data not shown). Twelve patients had a temporary increase of 1 or 2 points above their baseline on the two scales at any time during the study. To rule out the possibility of rater bias, such increases on both scales were examined; only three patients had a transient 1-point increase simultaneously on two scales.
Discussion
The combination of mirtazapine with fluoxetine, venlafaxine, or bupropion from treatment initiation was as well tolerated as fluoxetine monotherapy and produced greater improvement in depressive symptoms over a 6-week period.
This double-blind trial was designed to build on a previous study that examined the efficacy of mirtazapine, paroxetine, and their combination from treatment initiation (
14). There was no placebo arm in either study because the main goal was to surpass standard of care, which is antidepressant monotherapy. Knowing that all patients received at least one active treatment could have contributed to an overestimation of improvement. However, the remission rate among patients receiving fluoxetine monotherapy was only 25%, the same as for mirtazapine and paroxetine monotherapy in the earlier study (
14). In the control group, the dose of fluoxetine was not optimized, but neither were the regimens of the other agents, with the exception of venlafaxine, which was titrated in order to reliably inhibit norepinephrine reuptake (
15,
20). It is noteworthy that in the previous mirtazapine-paroxetine trial, an optimization step for the monotherapies after 4 weeks did not produce additional benefits, while the maintenance of the initial doses of the two drugs used in combination clearly produced additional therapeutic benefits (
14).
Given the sedative property of mirtazapine and its association with potential weight gain, we selected more activating and weight-neutral antidepressant drugs than paroxetine to combine with mirtazapine (
27). Although few patients gained more than 7% of their body weight during the acute phase (Figure 2), there was a mean significant weight gain in the mirtazapine groups and none at all in the fluoxetine monotherapy group. In contrast, there was no overall weight gain in the prolongation phase in patients receiving mirtazapine, and only one patient in the mirtazapine groups dropped out because of weight gain. The use of anxiolytics/sedatives was minimal and similar in all groups but somewhat less in the group receiving combination treatment with mirtazapine and venlafaxine.
In this study, the universally accepted remission criterion of a score of 7 or less on the HAM-D was selected because there has been some debate as to the exact score on the MADRS that should correspond to remission (
22,
28). While the combination groups did not show statistically significant improvement in response rates, they clearly showed significant improvement in remission rates. Not only is remission the goal of treatment for major depression, but achieving it as early as possible is of critical importance (
29). Based on remission rates, the combination treatments yielded a number needed to treat of 3 to 5 over fluoxetine monotherapy, which is similar to the advantage of clozapine over conventional antipsychotics in the treatment of schizophrenia (
30).
The combination of mirtazapine and venlafaxine seemed to perform particularly well; the group receiving this treatment achieved the highest remission rate, had the highest number of patients with a score of 1 on the CGI severity scale, and had no dropouts attributed to lack of efficacy. This was possibly due to the synergies between serotonin (5-HT) and norepinephrine reuptake inhibition by venlafaxine and 5-HT
2A/2C receptor antagonism, as well as blocking inhibitory α
2-adrenoceptors on the cell bodies and terminals of norepinephrine neurons and on 5-HT terminals by mirtazapine (
15,
21,
31–
33). These observations suggest that the greater the number of neuronal elements recruited to enhance 5-HT and norepinephrine transmission, the greater the potential therapeutic benefit. Clinically meaningful differences between combination treatments could be established in the future only with larger numbers of patients in each arm.
The combination treatments appeared to produce a more rapid improvement than did fluoxetine alone (Figure 1). However, the sharper decline in symptom severity with the combination treatments was mostly a reflection of having more patients achieve remission within the 42 days of the trial. Indeed, in the time to response and the time to remission, no difference was observed between the fluoxetine monotherapy group and the combination treatment groups. Consequently, the use of these combinations in a 6-week window produced evidence for a more robust antidepressant effect but not for a more rapid onset of action. The concomitant use of two drugs at treatment initiation still appears to be a more time-efficient approach to achieve remission than a substitution or an augmentation (
8–
12).
In the previous mirtazapine-paroxetine trial, it was observed that remission was sustained over 4 months in 90% of the patients receiving the combination (
14). In the present study, the double-blind discontinuation of one agent produced a 40% relapse rate, which suggests that the combination was important in these patients. There seemed to be fewer relapses among patients receiving bupropion than among those receiving fluoxetine or venlafaxine combinations. This might be attributable to fewer patients having been ill for more than a year and fewer with melancholia in the bupropion combination group than in the other two groups (Table 1). Notably, when a combination was reinstated after discontinuation, almost all patients promptly regained their prior improvement. It is also interesting to note that the time to response during the acute phase was not predictive of relapse in the prolongation phase.
The observations in the prolongation phase have practical implications for the use of antidepressant combinations from treatment initiation. Not only is the approach as well tolerated and more efficacious than monotherapy, but if a drug becomes intolerable due to side effects or if the combination becomes a financial burden, one drug could be stopped. In fact, more than half the patients in the 6-month prolongation did not relapse. Using combination therapy from the start is already in practice in other branches of medicine (e.g., β-adrenoceptor agonists plus steroids for asthma) and represents the standard of care for some psychiatric disorders (e.g., antipsychotics and mood stabilizers for mania).
The results of this study, taken together with those of three prior double-blind studies (
14,
34,
35), provide mounting evidence that combination therapy from treatment initiation provides superior clinical effectiveness in the treatment of major depression.