Sleep problems are a common and significant symptom of psychiatric illness (
1). Evidence from the past two decades suggests that the sleep problems associated with psychiatric illnesses may be more than just symptomatic consequences of the underlying illness. Rather, the sleep disturbances associated with psychiatric illnesses may be integral to the underlying disease process (
1–
8). If this is the case, appropriate treatment of the sleep disturbance associated with the psychiatric illness would involve much more than symptom management, since treatment of the sleep problem may be essential to the full promotion of recovery. For cocaine dependence, this possibility may be particularly important. There is currently no Food and Drug Administration (FDA)-approved medication for the treatment of cocaine dependence despite many efforts toward this end, and thus the identification of a novel target for medicinal therapy, such as sleep architecture, could have far-reaching clinical implications.
Cocaine dependence is associated with severe disruptions in sleep, during both periods of use and, perhaps more surprisingly, extended abstinence (
9). Early and influential studies of the subjective effects of cocaine withdrawal found that cocaine users reported poor sleep and fatigue in the first few days of abstinence but normal sleep shortly thereafter (
10,
11). However, small polysomnographic studies of sleep suggested that the sleep disturbance associated with abstinence from chronic use worsened rather than improved after initial abstinence (
12–
17). In the largest polysomnographic study to date (
18,
19), using laboratory cocaine administration and an inpatient setting to confirm abstinence, we demonstrated that the quality of sleep, measured polysomnographically, deteriorates from the first to third week of abstinence, with characteristic alterations in sleep architecture.
The changes in sleep architecture associated with chronic cocaine use include increases in sleep latency; decreases in total sleep time, slow-wave sleep time, and slow-wave activity; and alterations in REM sleep (
12–
20). These deficits are present initially and worsen over the course of 3 weeks of abstinence (
18,
19), without signs of improvement, and are associated with sleep-related cognitive deficits (
18) that may be sleep-stage dependent (
19). The sleep deficits are likely the consequence of long-term use of cocaine and could increase the likelihood for relapse (
21,
22). The concurrent improvement in self-reported sleep quality (
18), consistent with earlier studies (
10,
11), may contribute to these deficits escaping recognition as clinically relevant. Thus, the term "occult insomnia" was conceived to describe the discordance between self-reported and objectively measured sleep quality (
18).
Intriguingly, one class of candidate pharmacotherapies for the treatment of cocaine dependence may positively affect sleep. These medications enhance γ-aminobutyric acid (GABA) neurotransmission and include vigabatrin (γ-vinyl GABA), tiagabine, and topiramate (
23). For example, a small study has shown that tiagabine dramatically increases slow-wave sleep (sleep stages 3 and 4) in chronic cocaine users (
24) at the expense of lighter sleep stages (sleep stages 1 and 2). Benzodiazepines (GABA active sedative/hypnotic agents that may be clinically harmful in the treatment of cocaine dependence) have the opposite effect, increasing time in the lighter sleep stage 2 (
24), which suggests that the effects of these medications on sleep architecture may predict their clinical effectiveness. However, what may be the most promising medication (
23,
25) for the treatment of stimulant dependence—modafinil—is not considered to be in the same class as vigabatrin, tiagabine, and topiramate, nor does it appear to have effects on sleep architecture in the populations in which it has been shown to be clinically effective (i.e., its effects on narcolepsy, obstructive sleep apnea, or shift work-related sleep disorder) (
26–
28).
Modafinil is a wakefulness promoting agent with effects on dopamine neurotransmission (
29–
33) and apparent effects on glutamate and other neurotransmitters (
34). Modafinil is viewed as an abstinence initiation medication that may offset acute withdrawal symptoms (
25), whereas the GABA active class of medications may reduce the reinforcing effects of cocaine and thus reduce the propensity for relapse. However, the mechanisms whereby any of these medications may help to reduce cocaine use are currently unknown. In prior investigations (
18,
19,
24), we hypothesized that normalizing the aberrations in sleep architecture associated with abstinence may contribute to the efficacy of treatments for cocaine dependence, and we predicted that modafinil would have previously unrecognized positive effects on sleep and sleepiness in chronic cocaine users.
In the present study, we examined the effects of modafinil on objective and subjective measures of sleep and sleep-related outcomes in abstinent cocaine users. We hypothesized that morning-dosed modafinil would decrease nocturnal sleep latency, increase total sleep time, and decrease daytime sleepiness.
Results
Demographic Characteristics
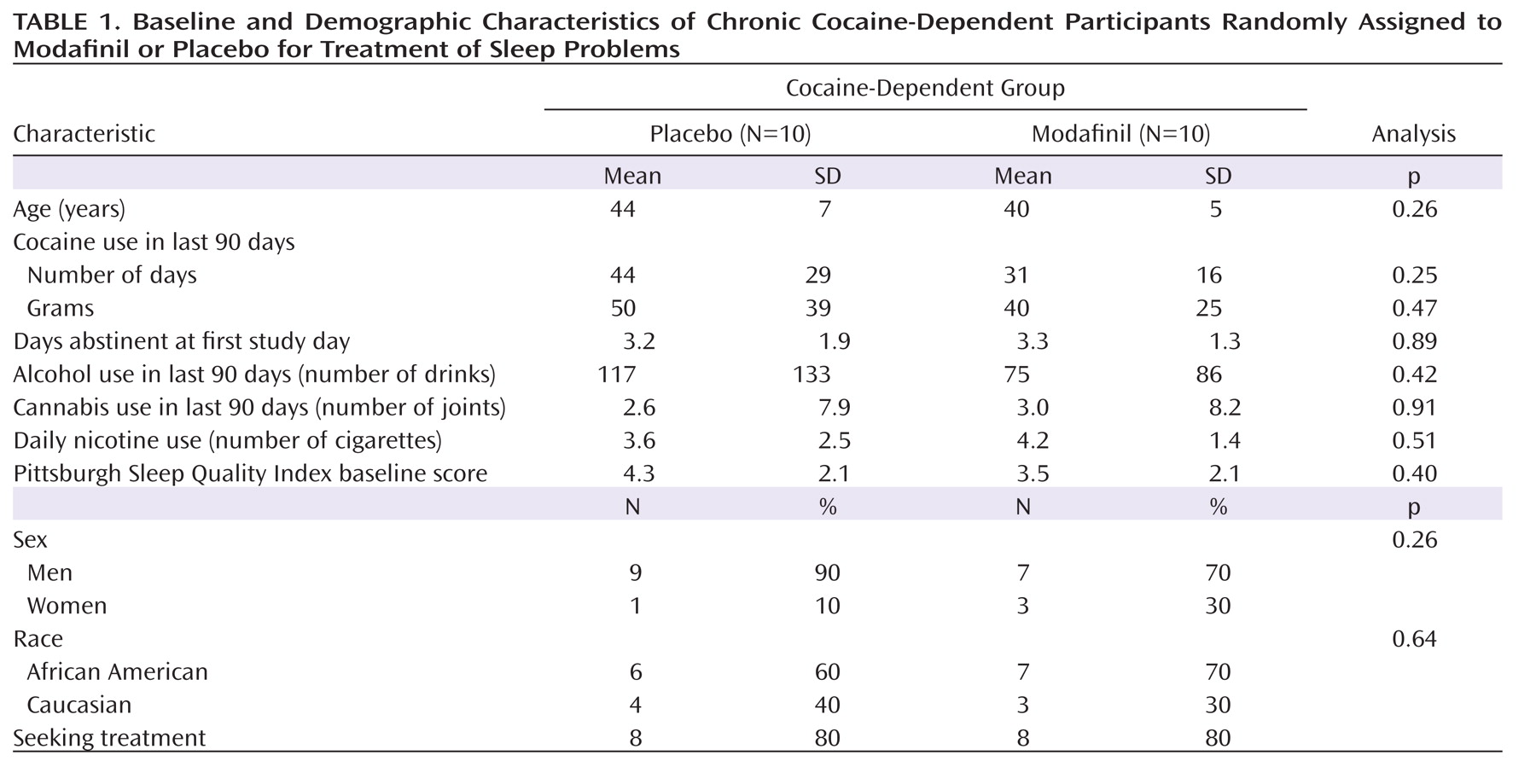
Demographic data for cocaine-dependent participants are presented in
Table 1. There were no statistically significant differences between the modafinil and placebo groups with regard to age, gender, and race. Self-reported cocaine, alcohol, cannabis, and cigarette use were similar between these two groups as well as the self-reported number of days abstinent from cocaine on day 1 of the study, which was the first day of modafinil administration.
Polysomnographic Sleep Measurement
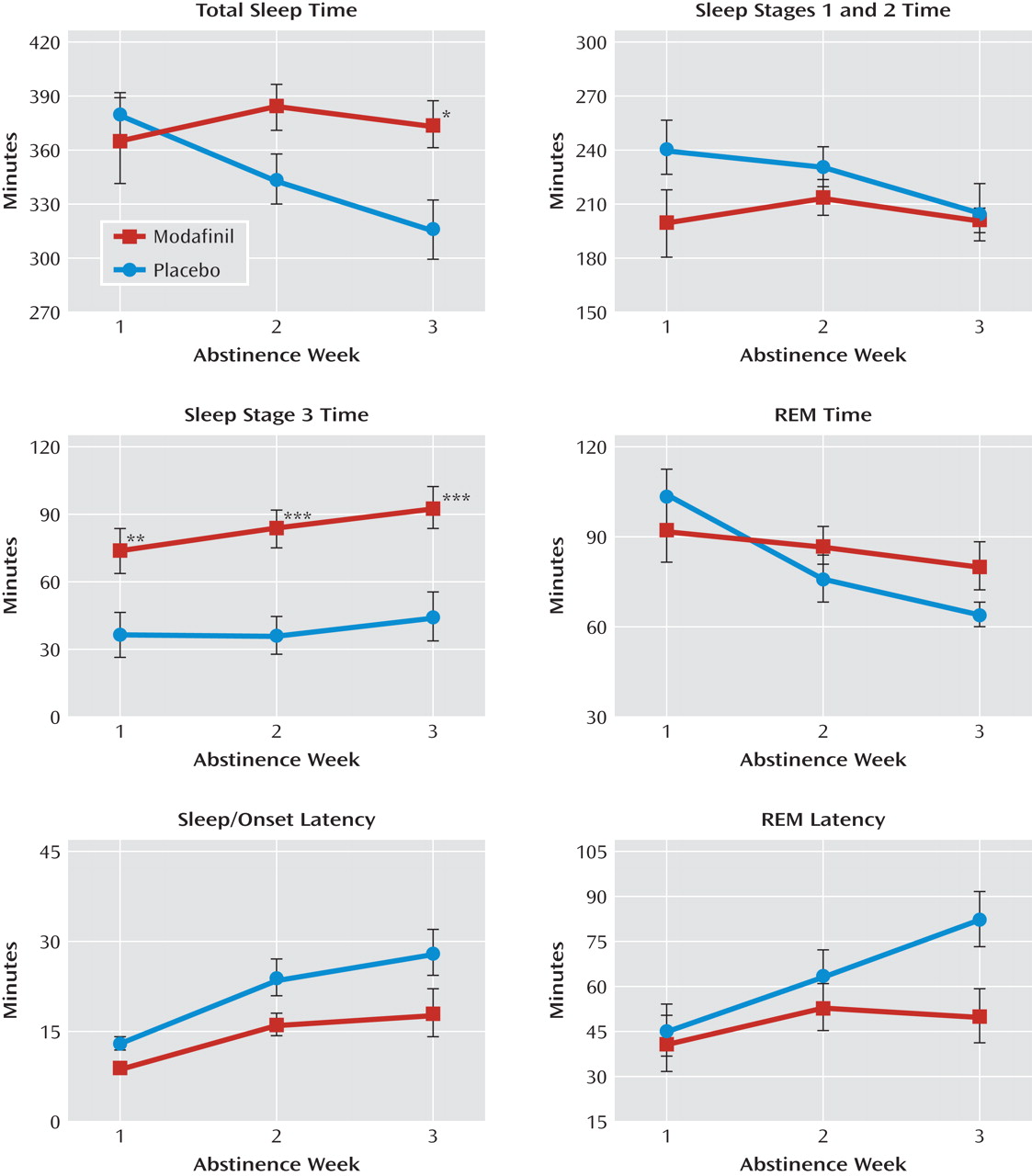
Polysomnographic sleep data are illustrated in
Figure 1. There were main effects of modafinil on sleep-onset latency (F=5.8, df=1, 18, p=0.03) and time spent in sleep stage 3 (F=11.9, df=1, 18, p=0.003). In addition, there were main effects of abstinence week on all assessed polysomnographic sleep measures as follows: total sleep time (F=4.1, df=2, 36, p=0.03), sleep latency (F=20.0, df=2, 36, p<0.0001), time in sleep stages 1 and 2 (F=5.2, df=2, 36, p=0.01), time in sleep stage 3 (F=6.3, df=2, 36, p=0.005), REM sleep time (F=8.4, df=2, 36, p=0.0002), and REM latency (F=11.1, df=2, 36, p=0.001). Post hoc differences between abstinence weeks 1 and 3 reflected increases in sleep-onset latency (t=–4.9, df=36, p<0.0001), time in sleep stage 3 (t=–3.5, df=36, p=0.001), and REM latency (t=–4.0, df=36, p=0.0003) and decreases in total sleep time (t=2.8, df=36, p=0.008) and REM sleep time (t=3.0, df=36, p=0.005).
Significant interactions were observed between drug condition and abstinence week in total sleep time (F=7.4, df=2, 36, p=0.002), REM latency (F=3.6, df=2, 36, p=0.03), and REM sleep time (F=3.5, df=2, 36, p=0.04). There was a nearly significant difference in the interaction in sleep time for sleep stages 1 and 2 (F=2.6, df=2, 36, p=0.09). Post hoc assessment of these interactions revealed increases in total sleep time (F=6.7, df=1, 36, p=0.01) and decreases in REM latency (F=6.9, df=1, 36, p=0.01) as the result of modafinil treatment at week 3. No treatment differences were observed in REM sleep time at any time point.
The Multiple Sleep Latency Test
There were no main effects of modafinil or abstinence week on daytime sleep latency. However, there was a significant interaction between abstinence week and drug condition, with modafinil associated with longer mean sleep latency (modafinil, 15 minutes [SD=2]) versus placebo, 10 minutes [SD=2]) during the first week of abstinence only (F=4.4, df=1, 36, p=0.04).
Subjective Sleep, Alertness, and Sleepiness Measures
Participants reported progressively improving subjective sleep and alertness with abstinence. The following improvements were statistically significant: main effects of abstinence week on overall sleep quality (F=9.0, df=2, 36, p=0.0007), depth of sleep (F=6.3, df=2, 36, p=0.004), feeling well-rested upon awakening (F=5.9, df=2, 36, p=0.006), alertness upon awakening (F=8.3, df=2, 36, p=0.001), and retrospective daytime alertness (F=7.2, df=2, 36, p=0.0023). There were no statistically significant effects of modafinil on any of these measures.
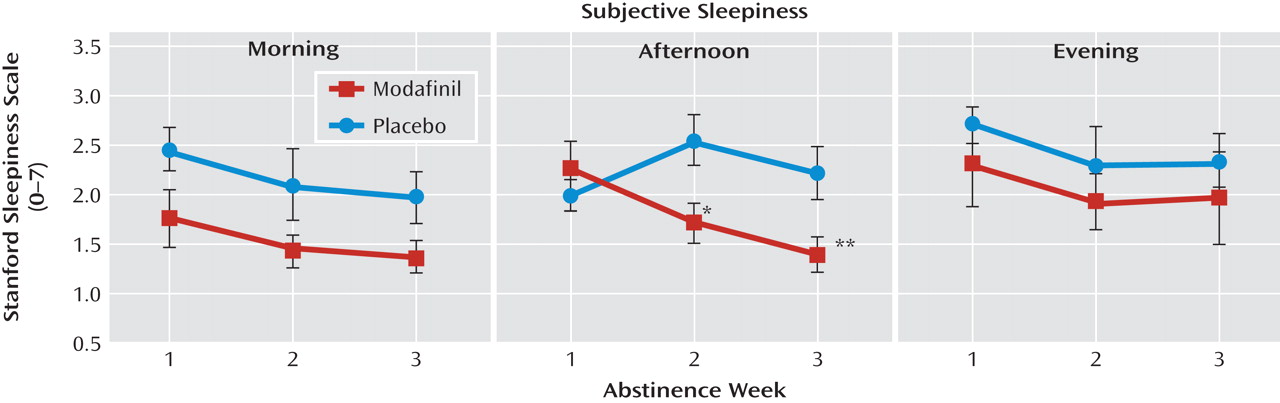
Results from Stanford Sleepiness Scale measures, assessed in the morning, afternoon, and evening, are illustrated in
Figure 2. Nearly significant differences were observed for drug condition (F=3.4, df=1, 144, p=0.07) and abstinence week (F=3.0, df=2, 144, p=0.06). There was a main effect for time of day (F=5.1, df=2, 144, p=0.007) and a significant interaction among drug condition, abstinence week, and time of day (F=2.7, df=4, 144, p=0.04). Statistically significant post hoc comparisons revealed an association between modafinil and decreased sleepiness during morning and afternoon testing at weeks 2 and 3. No modafinil effects were observed for evening sleepiness. Sleepiness at abstinence week 3 was less than at abstinence week 1 (t=2.4, df=144, p=0.02).
Comparisons With Healthy Participants
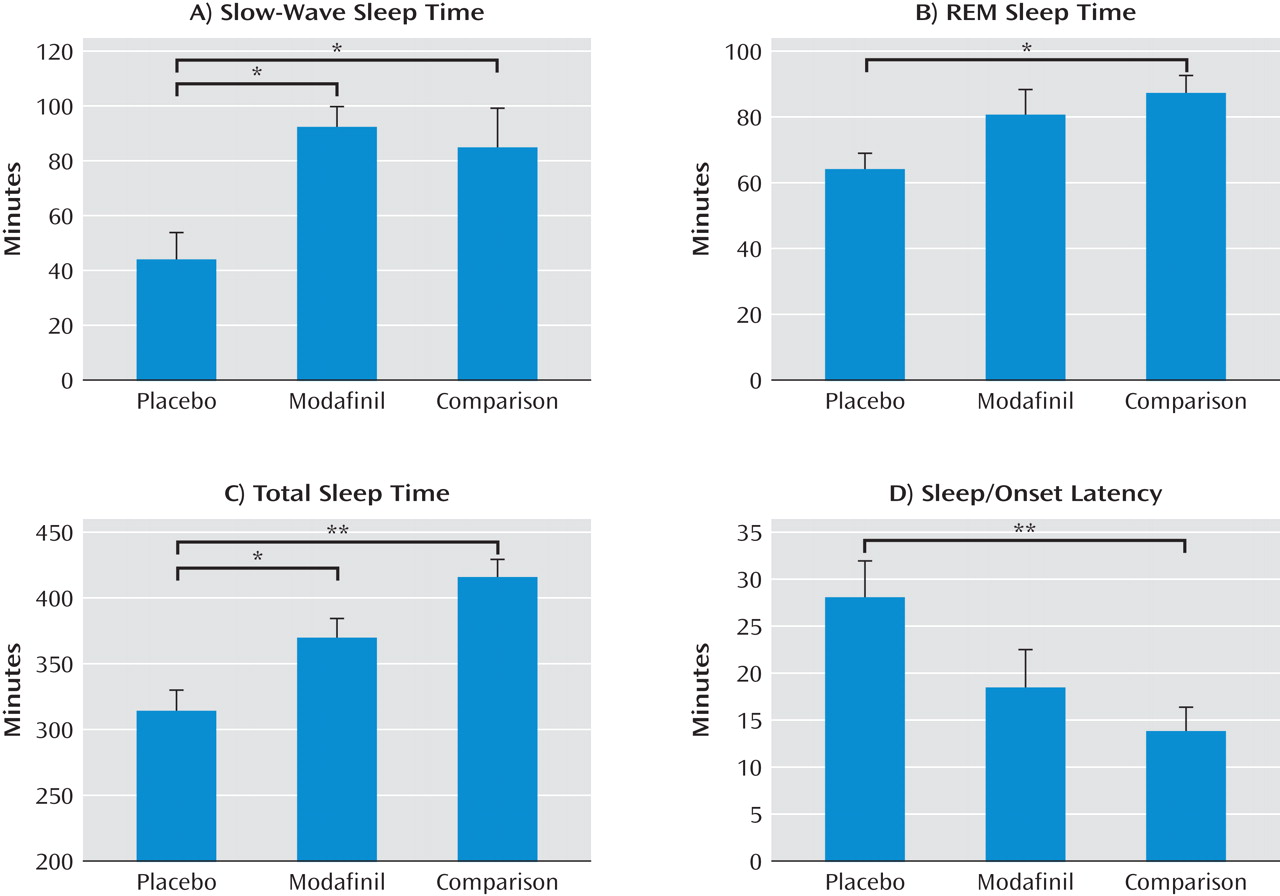
Compared with cocaine-dependent participants (Table 1), healthy comparison participants were similar in age (39 years [SD=9]), had nonsignificantly lower baseline Pittsburgh Sleep Quality Index scores (3 [SD=1.5]), and drank significantly less alcohol (19 drinks in the past 90 days [SD=15], p<0.05). Data for healthy comparison participants on polysomnographic- and subjective-measured sleep were compared with these data for cocaine-dependent participants in the following three-group comparison: healthy comparison group, cocaine-dependent group receiving placebo, and cocaine-dependent group receiving modafinil. Data from abstinence week 3 were used in the assessment of cocaine-dependent participants. There were statistically significant group differences in slow-wave sleep (sleep stage 3) time (F=4.9, df=2, 29, p=0.02), REM sleep time (F=3.5, df=2, 29, p=0.04), total sleep time (F=12.1, df=2, 29, p<0.0001), sleep-onset latency (F=4.7, df=2, 29, p=0.02), time in sleep stages 1 and 2 (F=3.6, df=2, 29, p=0.04), and REM latency (F=3.5, df=2, 29, p=0.04). There was no statistically significant difference in self-reported overall sleep quality (cocaine-dependent placebo group, 77 [SD=5]; cocaine-dependent modafinil group, 81 [SD=5]; healthy comparison group, 67 [SD=6]). Post hoc comparisons showed statistically significant normalizing effects of modafinil on slow-wave sleep time and modafinil-associated decreases in differences in REM sleep time, total sleep time, and sleep-onset latency between the placebo-treated group and healthy comparison group (
Figure 3). REM latency was not significantly different between either of the cocaine-dependent groups and the healthy comparison group, and there were no post hoc differences in the time in sleep stages 1 and 2.
Adverse Events
Six cocaine-dependent participants receiving modafinil and two receiving placebo experienced a total of nine adverse events. Four participants receiving modafinil and one receiving placebo reported headaches. Two participants receiving modafinil reported nausea without vomiting. One of the participants who experienced nausea also experienced palpitations and was evaluated in the local emergency department and had the dose of modafinil lowered on days 3 and 4 before returning to the full dose (
38). One participant receiving placebo developed conjunctivitis (possibly related to the polysomnographic set-up procedure). All of the other adverse events were considered mild and did not require medical intervention or reduction in the modafinil dose.
Discussion
To our knowledge, this is the largest study of polysomnographically measured sleep in chronic cocaine users to date and the first study of the effects of modafinil on sleep in this population. The most intriguing finding is the dramatic and therapeutically suggestive effect of modafinil on objective measures of sleep and daytime sleepiness in chronic cocaine users. Additionally, these results confirm earlier studies showing severe disruptions of sleep in abstinent cocaine users and a lack of subjective awareness of those disruptions.
Previous polysomnographic studies (
12–
16,
18,
19,
24) suggested that cocaine-related sleep disturbances extend beyond the acute and early withdrawal effects of cocaine (
10,
11). In the present study, we report that abstinence from chronic cocaine use is indeed associated with disruptions of sleep that are present within the first week of abstinence (e.g., disruptions in sleep stage 3 time) and worsen over 3 weeks of abstinence (e.g., disruptions in sleep-onset latency, REM sleep time, time in sleep stages 1 and 2, total sleep time). However, prior studies that used only self-reported sleep measures typically reported improvement in sleep during abstinence (
10,
11,
43). In a previous investigation (
18), we demonstrated this discrepancy explicitly and found that polysomnographically measured sleep and sleep-related cognitive performance deteriorated over 2 to 3 weeks of abstinence, while self-reported sleep quality improved. These findings were interpreted as a possible dysregulation of the homeostatic sleep drive and were termed "occult insomnia." The present findings reproduce our previous findings in both the objective deterioration and subjective improvement in sleep quality during abstinence. However, the novel findings of the present study are the striking effects of modafinil on sleep latency, sleep duration, sleep architecture, and daytime sleepiness.
We found that modafinil, 400 mg, given in a single, early morning dose, leads to improvements in sleep and decreases in objective and subjective measures of daytime sleepiness. The improvements in sleep occurred as either overall drug effects (sleep latency decreased and time in sleep stage 3 increased) or interactions with abstinence week, where the improvements reflected a reversal of the deterioration that developed over 3 weeks of abstinence in placebo-treated participants (in total sleep time and REM sleep time).
The modafinil-related improvement in objective daytime sleepiness, as measured by the Multiple Sleep Latency Test, was present only in abstinence week 1, when excessive somnolence is a well-known clinical feature of cocaine withdrawal (
10). One interpretation of this finding is that modafinil's dopaminergic effects (
31–
33) on wakefulness are more apparent during the relatively hypodopaminergic state of early abstinence from cocaine. This interpretation is consistent with an overall decreased propensity to sleep (i.e., increased sleep-onset latency and decreased total sleep time) in the second and third weeks of abstinence. Subjective sleepiness was also improved with modafinil, with statistically significant effects 2.5 and 8 hours after dosing but no effect 13.5 hours after dosing. Unlike objectively measured sleepiness, these effects were observed in the second and third weeks of abstinence. This difference may reflect several factors. As mentioned earlier, the propensity toward sleep diminishes as abstinence progresses, and therefore the objective effect of modafinil on reducing daytime sleep latency may be limited. However, subjective symptoms of reduced sleep time, such as diminished cognitive performance, may worsen with continued abstinence (
18) and thus may be more responsive to modafinil treatment as abstinence progresses. The absence of a subjective effect of modafinil on nighttime sleepiness may reflect the lower concentration of modafinil at that time (modafinil half-life: approximately 10 to 15 hours [
44]) and is consistent with its positive, objective effects on nocturnal sleep latency and sleep architecture.
The strongest effect of modafinil on sleep architecture appears to be the increase in the time in sleep stage 3 (slow-wave sleep). Slow-wave sleep was significantly higher in the modafinil group, more than double that of the placebo group, at all time points. From a clinical perspective, this effect may also be particularly relevant. Slow-wave sleep is a highly protected stage of sleep. In healthy individuals who are limited to 4 or 6 hours in bed, slow-wave sleep time is not reduced (
45) and is dramatically increased following total sleep deprivation (e.g., reference
46). In addition, there is evidence that increasing slow-wave sleep time may improve cognitive performance (
47). Nevertheless, slow-wave sleep time in chronic cocaine users is markedly decreased (
18), and deficits in slow-wave sleep are also associated with chronic use of other addictive substances (e.g., reference
48). Indeed, the observed deficits in slow-wave sleep may be necessary to see such an effect from modafinil, since large studies of modafinil in other populations have not reported such effects (
26–
28). However, stimulant effects on slow-wave sleep are not unprecedented, since, for example, cocaine given early in the day increases nocturnal slow-wave activity (
18).
The present study suggests that modafinil exhibits its greatest objective effects on sleep and sleepiness when the inherent abnormality is greatest. Hence, objective daytime sleepiness was improved with modafinil during the first week of abstinence (when sleepiness is greatest), and sleep latency decreased and total sleep time increased with modafinil later in abstinence when these measures were most abnormal. Although slow-wave sleep increased somewhat with abstinence, deficits in slow-wave sleep time in cocaine-dependent participants were prominent throughout abstinence, and the increase in slow-wave sleep caused by modafinil was similarly stable across the study. Consistent with the notion that modafinil reverses sleep deficits, modafinil appeared to normalize sleep in cocaine-dependent participants relative to healthy comparison participants. Indeed, modafinil-treated cocaine-dependent participants had normal slow-wave sleep time, a significant improvement in total sleep time, and apparent reductions in deficits in REM sleep time and sleep-onset latency. In contrast, self-reported sleep quality among cocaine-dependent participants (both modafinil- and placebo-treated) was not significantly different from that among healthy comparison participants, nor was it numerically better than that among healthy comparison participants, supporting the previous finding of "occult insomnia" (
18).
Strengths of the present study, compared with previous polysomnographic studies of sleep in cocaine users, are the relatively large number of participants and the use of a fixed time in bed for all participants. However, the number of participants was still small for a placebo-controlled trial and was powered only to detect the previously observed changes in sleep during abstinence in each group separately (
18,
19,
24). Another limitation of the study design was the lack of occipital EEG leads for nocturnal polysomnography. Although this limitation may not have affected the experimental groups differently, it may have caused a small systematic difference in the observed nocturnal sleep latencies. In addition, the use of the fixed time in bed, while a strength, introduced the possibility of an unforeseen group difference. In particular, some participants may have had more difficulty adapting to the 11:00 p.m. bedtime than others, and formal assessment of "morningness/eveningness" was not conducted (and possible differences between groups in "morningness/eveningness" are not known). However, the sleep results for the placebo group of this study match well with our prior study in which bedtime was flexible (
18,
19), suggesting that any difficulty adapting to the fixed schedule did not likely affect this aspect of the results. Although cocaine use immediately prior to the start of the study was not normalized through the use of laboratory cocaine administration, the accuracy of observed urine toxicology screens at the time of admission provided reasonable assurance that all participants were between 2 and 6 days of abstinence, consistent with their self-report. Other limitations include the lack of control over daytime lighting conditions, the lack of a substantial number of nontreatment seeking cocaine users, and the possibility of withdrawal from other substances influencing the measured outcomes. The latter two concerns were largely addressed in our previous studies, where we found similar changes in sleep during abstinence in nontreatment seeking participants, and in a study design that controlled for withdrawal from other substances (
18,
19).
The present results suggest that modafinil, given early in the morning, has positive effects on sleep and sleepiness in chronic cocaine users. In early abstinence, modafinil decreases the objective and subjective sleepiness associated with withdrawal from cocaine. Throughout 3 weeks of abstinence, it decreases subjective afternoon sleepiness. Nocturnally, latency to sleep onset is decreased with modafinil and sleep architecture is improved, with severe deficits in slow-wave sleep reversed and the development of REM sleep and total sleep time deficiencies during abstinence reduced. These effects are likely mediated in large part by the cocaine-like effects of modafinil on dopamine neurotransmission (
29–
33,
49). Cocaine given approximately 9 hours prior to sleep, for example, has similar effects, including an increase in slow-wave sleep activity on the same night and rebounds in REM sleep and total sleep time that develop the next day (
18,
19). We hypothesize that the apparent clinical effectiveness of modafinil given in the morning (
25) is in part due to these effects on daytime sleepiness and nocturnal sleep. Finding such a connection between sleep improvement and clinical effectiveness in cocaine dependence, a condition that is not intrinsically a sleep disorder, is also relevant to the treatment of other conditions in which sleep abnormalities may contribute to morbidity, inhibit recovery, and, indeed, may be closely related to the disease process (e.g., major depression [
2]). This connection should be explicitly and prospectively tested in a controlled clinical study.