Clinical management of antipsychotic drug treatment is increasingly complex, with growing numbers and applications of clinically employed agents, inconclusive findings among treatment efficacy and effectiveness trials involving first- or second-generation antipsychotic drugs, and polytherapy with complex drug combinations (
1–
12). Despite growing clinical and research requirements for reliable dosing estimates, available dosing guidelines rest on an inadequate research base (
2,
13–
18). Proposed schemes usually are based on averages of doses recommended by manufacturers and approved by regulatory bodies or on expert-estimates of approximate clinically equivalent potency (
2,
13,
19). It would be desirable to base such potency comparisons on randomized, especially head-to-head, comparisons of a range of antipsychotics at several fixed doses, and across various diagnostic, illness severity, comorbidity, and demographic variables. However, currently available information arising from research of this kind is severely limited, and such studies remain rare (
17,
20,
21). Uncertainties about clinically equivalent doses of antipsychotics probably contribute to apparent variance in efficacy and tolerability in clinical practice and to inconsistent findings among experimental clinical trials (
4,
5,
22–
25).
Method
Participants
We identified colleagues with experience in clinical research and in the clinical use of antipsychotic drugs, based on preliminary literature searches, reputation among peers, and by investigator consensus. We sought geographically and demographically diverse participation. Research experts were considered eligible only if they had at least five peer-reviewed publications related specifically to clinical trials of antipsychotic drugs. Clinical experts were referred by participating research experts as particularly knowledgeable in the use of antipsychotic medicines, with extensive clinical practice experience.
Survey
The survey instrument included three sections: [A] clinically equivalent doses; [B] dosing recommendations; and [C] dosing adjustments for specific, defined, circumstances. In section A, participants were to estimate the clinically equivalent dose of 59 antipsychotic preparations, including 36 orally administered agents and 13 short-acting and 10 long-acting injected agents. Clinical scenarios used olanzapine, 20 mg/day, as the reference treatment for the equivalency estimates of the oral and long-acting injectable agents. We calculated the dosing ratios to this dose of olanzapine and to the equivalent dose for chlorpromazine as a historical reference. Injectable haloperidol, 5 mg, was the reference for short-acting injectable agents. Study participants also rated their confidence in each estimate provided, as high (based on extensive experience; applies to most patients), moderate (some experience; probably applies to most patients), or low (limited experience or frequent exceptions based on clinical circumstances). Participants were instructed to give priority to efficacy over tolerability in dosing estimates.
Section B requested usual starting doses, typical target ranges, and usual maximum doses for the each oral or injectable agent (N=61). Brief, standardized clinical vignettes provided a consistent context for responses. Typically, vignettes were based on treatment of adult males with DSM-IV or ICD-10 schizophrenia, with ≥2 years of lifetime exposure to antipsychotics and not considered treatment refractory or intolerant. Section C sought dosing recommendations based on variance in demographic and clinical circumstances.
Survey Methods
Following review and approval of the study protocol by the Dalhousie University Health Sciences Research Ethics Board, each study participant provided written, informed consent for participation and public acknowledgment. In 2007–2008, participants were sent the same study survey instrument electronically in two stages, based on the iterative Delphi method of consensus-building, a method that has been used extensively in social sciences and health research including mental health (
26–
28). This method provides for independent input by each participant at both stages, with anonymous, summary information provided at stage II to each participant based on the averaged, collective contributions from stage I. Summary information included the means (with standard deviations and coefficients of variation), medians (with ranges and interquartile ranges), and the median confidence levels of stage I responses regarding each drug, as well as the number of respondents per estimate. Minor adjustments for stage II included addition of clotiapine, as suggested by several participants, and removal of spiroperidol and pipothiazine undecylenate for lack of responses in stage I. We also modified section C to request dosing recommendations in the presence of common comorbid medical disorders (diabetes mellitus, ischemic heart disease, organic brain syndrome, substantial hepatic or renal impairment) identified by respondents in stage I.
Data Analysis
First, we evaluated associations of recommended oral doses averaged across all drugs as a continuous variable for each study participant versus a total of nine participant characteristics, including four continuous variables tested with linear regression (age, years of experience, antipsychotic research studies per participant, and peer-reviewed publications per participant), with contingency tables to evaluate four categorical variables (sex, country, majority of patients treated [in terms of diagnosis and race/ethnicity], and main career interest [research/clinical] of participants).
In addition, we sought recommendations for adjusting oral doses of antipsychotics based on the following patient demographic and clinical characteristics: sex, age, race or ethnicity, diagnosis, current clinical state, illness severity, illness duration, specific psychiatric comorbidity (substance use or anxiety disorders), medical comorbidity (ischemic cardiac disease, clinically hepatic or renal impairment, diabetes mellitus, delirium, or dementia), abnormally high or low body weight (based on standard BMI criteria provided), and type of oral formulation. For each factor, we evaluated the proportion of participants recommending a dose change and calculated median percentage changes in dose.
We summarized reported estimates of clinical dosing equivalents among agents and clinical circumstances, based on comparison to standard treatments, as reported in stage II. As median and mean oral dosage equivalency estimates were very similar in our preliminary assessment (r=0.997, p<0.0001; slope=1.01 [95% CI= 0.985–1.04]), only medians with interquartile range (IQR) are reported for simplicity and to limit the impact of potential extreme or outlier values. IQR represents the midpoint of the difference between the 25th and 75th percentile values. Coefficient of variation (SD/mean×100) was used to indicate consensus in final recommendations and was defined empirically as high (≤25%), moderate (26%–33%), or relatively low (>33%). For oral agents, equivalency ratios were calculated for olanzapine, the reference treatment, and for chlorpromazine. Statistical analyses employed commercial software (Stata.8®, Stata Corp, College Station, Tex.; Statview.V®, SAS Corp, Cary, N.C.; and SPSS 15.0 for Windows, Chicago).
Results
Participants
We obtained agreement to participate from 46 of 111 candidates (41.4%); 43 of these participants (93.5%; 32 researchers, 11 clinicians), representing a wide geographic distribution and 18 countries, completed both stages of the Delphi process. The participant group was mostly male (91%). The mean age of the group was 49 years (SD=10). Participants had a mean of 23 years (SD=10) of experience with antipsychotics and had been involved in a median of 15 studies with 30 antipsychotic peer-reviewed publications. For their primary interest, 58% specifically indicated schizophrenia, with 35% more broadly indicating psychotic or major mood disorders. Their experience was primarily with Caucasian patients (72%), East-Asian (14%), or patients of various races (Asian, black, Caucasian including Hispanic, 14%).
Geographically, most respondents were from North America (U.S. [N=10] or Canada [N=4]) or Western Europe (Italy [N=4], Spain [N=4], Austria [N=2], Denmark [N=2], Germany [N=2], Portugal [N=2], Belgium [N=1], Finland [N=1], or United Kingdom [N=1]). Other regions included Japan (N=3), Turkey (N=2), and India, Israel, People's Republic of China, Republic of Korea, and Taiwan (N=1 each).
Delphi Effect and Consensus
Each stage of the Delphi process required participants to complete up to 59 clinical equivalency estimates and 191 dosing recommendations. The consistency of equivalency estimates improved as the average coefficient of variation decreased from 57% in stage I to 33% in stage II (paired t test between study stages: t=7.20, df=58, p<0.0001). Consensus also improved among dosing recommendations (starting, target, and maximum), with moderate or high consensus (coefficient of variation<33%) rising from 29% to 47% between stages I and II. Final consensus, based on moderate or high median levels of agreement (coefficient of variation<33%), was 76.3% overall for equivalency estimates (long-acting injected agents: 90%; oral agents: 83%; short-acting injected agents: 46%) and 67% for all dosing recommendations (oral agents: 69%; short-acting injected agents: 67%; long-acting injected agents: 60%). Median dosing recommendations (mg/day) were 5.8% lower in stage II, reflecting changes in 28.7% (N=74 of 258) of initial recommendations.
Clinically Equivalent Dose Estimates and Recommendations
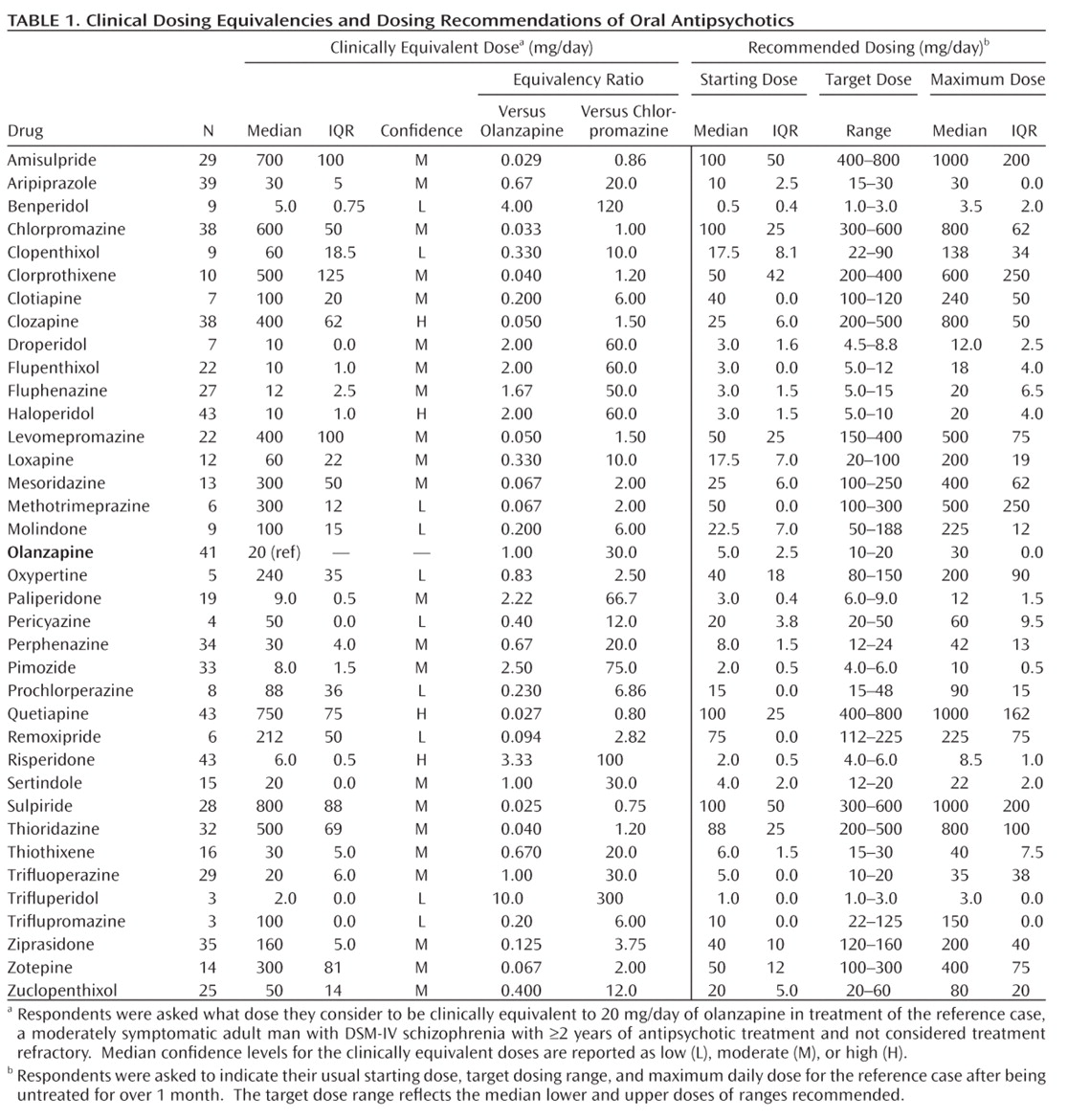
Clinically equivalent antipsychotic dosing recommendations were obtained for oral (N=36 [
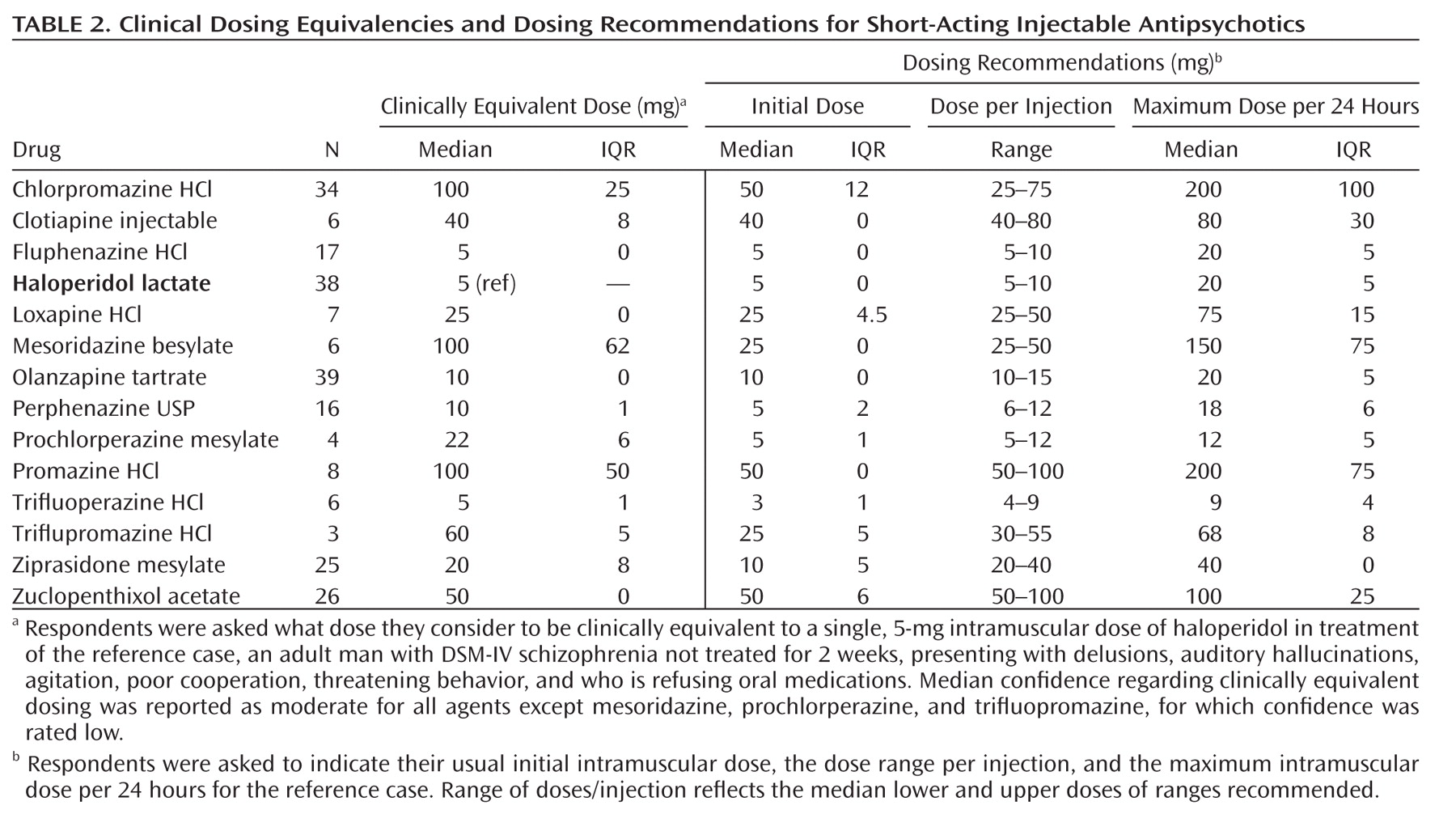
Table 1]), short-acting injectable (N=13 [
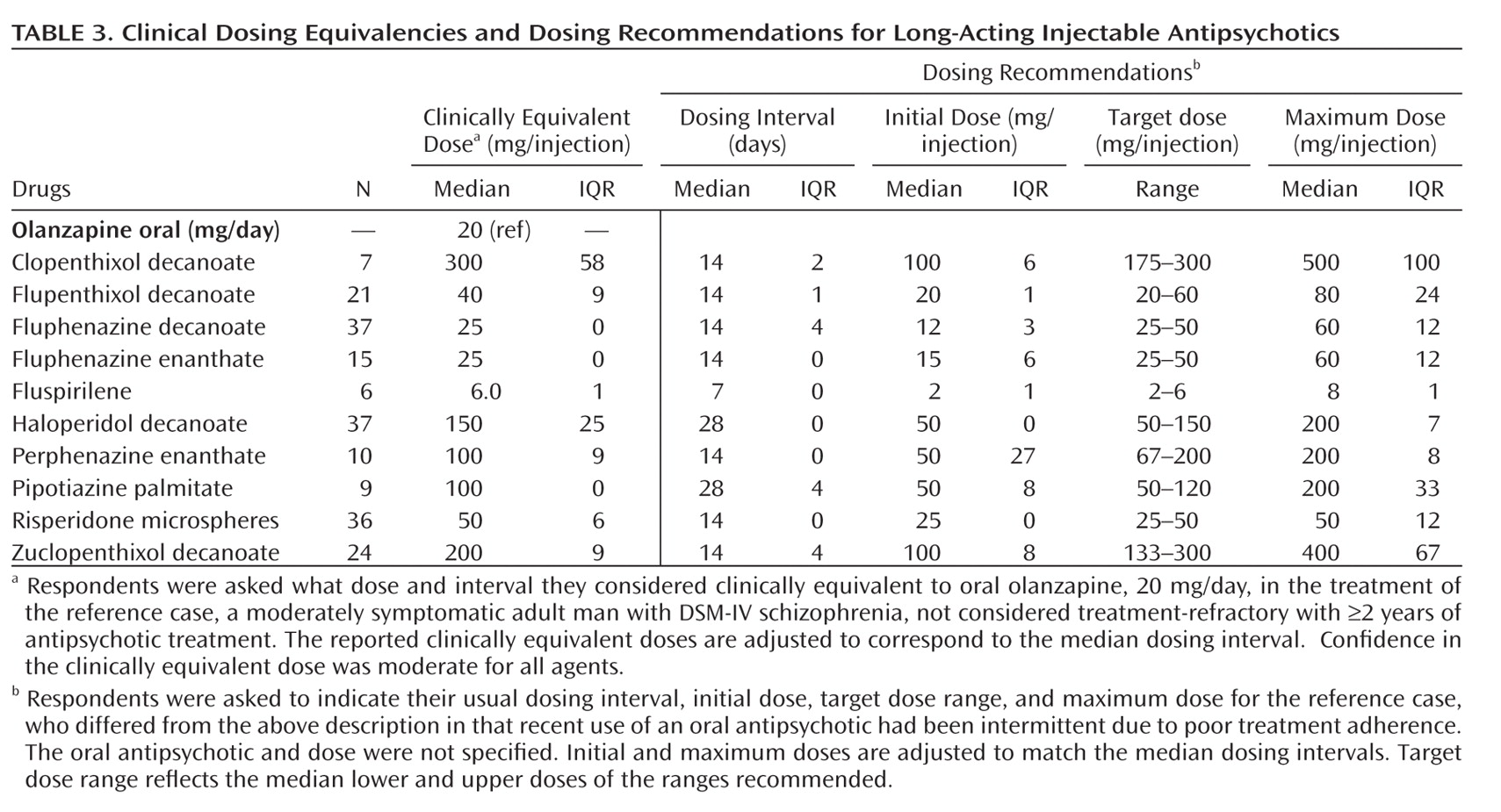
Table 2]), and long-acting injectable (N=10 [
Table 3]) formulations. Based on data reported (Table 1), the overall mean median starting dose for oral antipsychotics in olanzapine equivalents was 4.8 mg/day (IQR=1.4); the overall target dose range (mean median of lower and upper bounds of ranges) was 10.2 to 25.5 mg/day; and the overall mean median maximum dose for oral antipsychotics in olanzapine equivalents was 30.9 mg/day (IQR=7.5).
There were no significant differences in estimates of mean or median clinical dosing equivalences among all oral or parenteral antipsychotics between research (N=32) and clinical (N=11) experts. Overall, there was strong agreement between participant types regarding clinically equivalent dosing estimates for all agents and formulations (Pearson r=0.979). Findings were similar for dosing recommendations of oral agents (r=0.991) and for parenteral preparations (r=0.959; all p<0.0001).
Factors Associated With Dosing Recommendations
Preliminary bivariate analyses for effects on starting and maximum recommended daily oral doses related to eight participant characteristics (sex, age, years of experience, antipsychotic studies and publications/person, country, major diagnostic type of patients treated, and main career interest [research/clinical]) revealed no significant associations. Since no factor was sustained as important in these initial analyses (not shown) we did not pursue multivariate modeling.
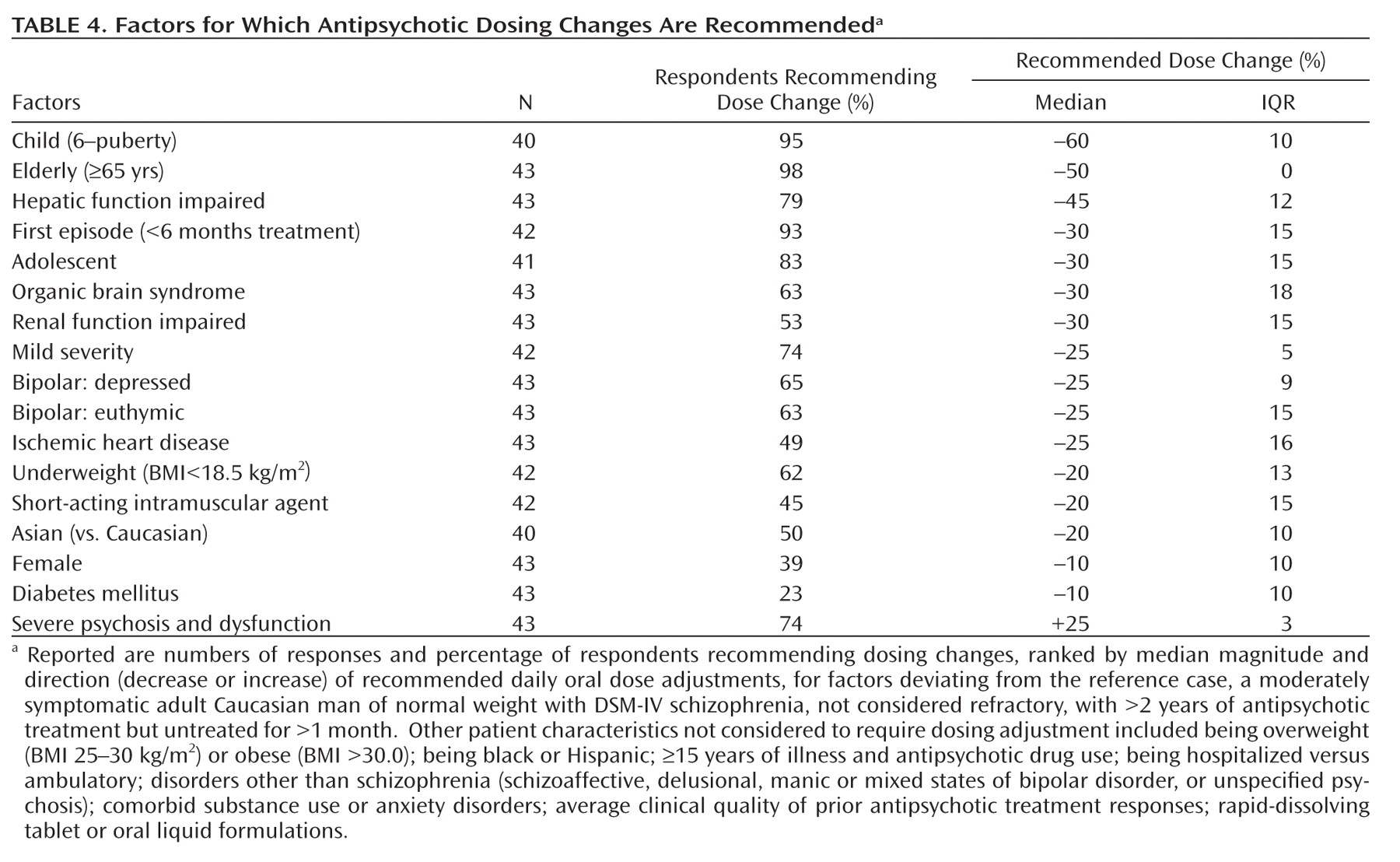
Several patient factors did affect dosing recommendations (
Table 4). As expected, age had a major effect in that recommended median daily oral antipsychotic doses were 60%, 30%, and 50% lower in children, adolescents, and the elderly, respectively, than in young adults. Selected medical comorbidities (e.g., diabetes, heart disease, hepatic or renal impairment, organic brain syndrome) also led to 10%–45% lower recommended median daily oral doses relative to adult patients without such illnesses. Respondents recommended an average of 20% lower daily antipsychotic doses for East-Asian versus Caucasian patients, even though dosing recommendations for the participant's predominant race/ethnicity of patients treated was not significantly associated with dosing recommendations (data not shown). Recommended daily doses averaged 10% lower for adult women than for men. For underweight patients, based on standard BMI criteria (<18.5 kg/m
2), recommended doses were 20% lower than for normal weight patients (BMI=18.5–24.9 kg/m
2) but were not affected significantly for overweight patients (BMI≥25 kg/m
2). Dosing recommendations were not affected by comorbid anxiety or substance use disorders.
Several factors related to psychiatric illness were associated with dosage modifications, although dosing was similar for most forms of psychotic or manic illness. However, bipolar depression or long-term prophylactic treatment of currently euthymic bipolar disorder patients called for dose reductions averaging 25% below the similar recommendations for acute mania or exacerbated schizophrenia. Severe, acute psychotic illness or a history of unsatisfactory treatment response led to recommended increases of daily antipsychotic dose by an average of 25%, whereas mild symptoms and first-episode of psychotic illness led to 25%–30% lower recommended doses than for repeatedly acutely psychotic patients, with no distinction between acute and chronic illnesses. There were no appreciable differences in recommended dosing for hospitalized versus ambulatory patients diagnosed with reasonably clinically stable, chronic schizophrenia. Drug formulations also had no effect on recommended doses, except that dosage reduction by 20% was recommended for short-acting injected preparations relative to oral preparations (Table 4).
Discussion
Broadly representative and plausible recommendations of clinically equivalent doses for antipsychotic drugs are needed to guide clinical dosing decisions, design of research studies, and interpretation of research findings (
2,
4,
5,
22–
25). Accordingly, this study employed a Delphi, two-stage, international survey of experienced and expert clinical and research colleagues to gain consensus estimates of clinically equivalent doses (compared to oral olanzapine or short-acting injectable haloperidol as standards). Dosing recommendations (starting dose, typical target range, and maximum daily doses) of currently clinically employed oral and parenteral antipsychotics also were sought using the same method along with consensus recommendations for their modification under specific clinical circumstances.
This is a first application of the otherwise widely employed Delphi method for seeking consensus to estimate equivalent doses of antipsychotics. Effectiveness of the two-stage Delphi method used is indicated by decreases in the variance of dose estimates between survey stages, and by achieving an average rate of 76% consensus in final (stage II) clinical equivalency estimates and 67% for all dosing recommendations.
Over several decades, there have been efforts to estimate equivalent doses of antipsychotic and other psychotropic drugs. Typically, these recommendations have considered limited ranges of agents and made use of findings from flexible-dose trials with varying ranges of permitted doses and clinically determined doses (
13–
18). Such recommendations risk confounding-by-indication and regression-to-mean artifacts and have led to sometimes strikingly inconsistent recommendations. Notably, the World Health Organization (WHO) system of expert consensus-based recommendations of defined daily doses for antipsychotics identified 10 mg/day of olanzapine as the average maintenance dose for adult patients diagnosed with schizophrenia (
18,
29); this is a conservative dose for many psychotic patients (
2,
3,
7). We used 20 mg/day of olanzapine as a standard reference treatment on which to base the reported clinically equivalent dosing estimates. Based on this difference in recommended doses of olanzapine, it is not surprising that the average equivalent dose among oral agents was 50% higher (1.5 ± 1.6 times greater) in this study than in WHO-defined daily dose recommendations, although these sets of equivalent doses are strongly correlated (r=0.920).
Fixed-dose, randomized trials involving multiple active comparators at a wide range of doses are required to estimate dosing equivalencies objectively and provide scientifically secure dosing recommendations. Considering the highly varied indications for antipsychotics and great heterogeneity of patients and their treatment responses, required studies would need to be either very large or repeated several times in dissimilar patient populations. Based on the limited available fixed-dose data, Davis and Chen (
17) attempted in 2004 to estimate the near maximal effective dose of antipsychotics by generating dose-response curves. Their findings demonstrate the limitations associated with this method. For oral antipsychotics, the computed near-maximally effective dose varied by 50% to 300% for only seven antipsychotics, with striking variance among individual dose responses for most drugs. For four agents they could only provide a threshold dose, with reference to which the near maximal effective dose was estimated to be above or below. For these drugs, limitations in the data failed to indicate at which dose the response curve plateaus. In only three cases were they able to report a single oral dose as the "near maximal effective dose" (amisulpride, 200 mg; aripiprazole, 10 mg; and risperidone, 4 mg). These estimates were based on findings with significant heterogeneity or minimal datapoints. With these limitations, it is not possible currently to determine clinically equivalent doses of antipsychotics firmly based on randomized, controlled, and blinded fixed-dose trials involving more than one drug, a placebo, and patients of specific types. This state of current knowledge is not surprising considering the heterogeneity of clinical presentations and of treatment responses, even among patients with nominally identical DSM or ICD diagnoses, long recognized by clinicians and researchers, and elegantly noted by Davis and Chen (
17). Despite these limitations and using the midpoint dose (estimated median) of the ranges reported by Davis and Chen (
17), we found a strong correlation between their near-maximally effective doses and the equivalent doses in our survey among 10 oral antipsychotics common to both investigations (Pearson's r=0.890, p<0.0001).
Other proposals to establish drug potency equivalents include use of plasma or serum concentrations of antipsychotic drugs and their major active metabolites, or modern brain imaging methods to estimate levels of receptor occupancy, usually involving competition for dopamine D
2 receptors labeled with positron-emitting, selective radioligands (
30–
32). Although theoretically attractive, such methods are costly and technically challenging. At best they provide indirect correlates to clinical response that may be especially questionable with respect to the limited D
2-antagonistic actions of some commonly used, second-generation antipsychotic agents (
2,
30–
33).
The present study identified several patient characteristics leading to recommended dosage adjustments (Table 4), notably including substantial lowering of doses for geriatric and pediatric patients, and increases for cases involving severe psychotic symptoms and dysfunction. These dosing recommendations appear to have face validity in that they are consistent with currently standard clinical practices. The specific consensus recommendation to reduce doses of antipsychotic drugs for East Asian patients by approximately 20% relative to Caucasians (Table 4) is consistent with some but not other reports concerning comparative dosing across races, and was not associated with the predominant race/ethnicity of patients usually treated by study participants, leaving the question unresolved (
25,
34,
35).
This study has several limitations. Although the range of geographic sites and professional experiences represented is quite broad, several regions of the world, especially Central and South America, much of Eastern Europe, and parts of the Middle East, Africa, and south-central Asia are notably unrepresented, and the sample comprised mostly male physicians despite efforts to recruit more women. A more substantive limitation is the lack of objective standards by which to select standard comparator doses, and to verify what are essentially clinical impressions, even though provided collectively and systematically by experts. Experience-based dosing opinions are susceptible to many influences, including uncritically accepted local dosing practices, inaccurate representations of personal practices, or inaccurate dosing recommendations by manufacturers in the regulatory approval process. In addition, the results assume, unrealistically, that clinical antipsychotic dosing is constant and uniform among diagnoses, under changing and typically unstable clinical conditions, and in often subtly differing forms or levels of symptomatic severity. In addition, several uncommonly used agents received relatively few responses from study participants (Table 1). Median doses and their variance are reported so as to minimize effects of outlier values, and they were remarkably similar to mean doses. Moreover, there is a tacit, but unrealistic, pharmacological assumption of linearity of dosing across a broad range of doses and across many dissimilar drugs that cannot be captured adequately with an estimated, consensus ratio of potency to a single, selected dose of a standard agent such as olanzapine or chlorpromazine (
2,
14,
17; Tables 1–3). Despite these and other limitations, we present the findings of this international survey as a representation of substantial and systematically acquired expert consensus. The findings should provide at least approximate and general guidelines to support clinical dosing in adults as well as planning and interpretations of experimental therapeutics trials and other research studies.
In conclusion, the reported findings arise from a large, systematic, international survey using Delphi methods to develop consensus. It provides general guidelines for approximately clinically equivalent recommended doses of a broad range of clinically employed, modern and older antipsychotics, including both oral and parenteral formulations. The study also provides recommendations from clinical and research experts on modification of dosing based on specific demographic or clinical characteristics of patients for whom antipsychotics are commonly employed.
Acknowledgments
The authors thank study participants (all of whom consented to be named): Drs. A. Carlo Altamura (Italy), Jose A. Apud (U.S.), A. George Awad (Canada), Ross J. Baldessarini (U.S.), Corrado Barbui (Italy), Daniel Casey (U.S.), Franca Centorrino (U.S.), Siow-Ann Chong (Singapore), Young-Chul Chung (Republic of Korea), Benedicto Crespo-Facorro (Spain), John M. Davis (U.S.), Marc De Hert (Belgium), W. Wolfgang Fleischhacker (Austria), David Gardner (Canada), John Geddes (United Kingdom), Birte Glenthoj (Denmark), Donald Goff (U.S.), Ricardo Gusmão (Portugal), Giuseppe Imperadore (Italy), Philip G. Janicak (U.S.), Yasuhiro Kaneda (Japan), Alain Labelle (Canada), Hsien-Yuan Lane (Taiwan), Stefan Leucht (Germany), John Luo (U.S.), Stephen R. Marder (U.S.), Massimo Mauri (Italy), Vicente Molina (Spain), Dieter Naber (Germany), Evaristo Nieto (Spain), Kensuke Nomura (Japan), Dost Öngür (U.S.), Linda Peacock (Denmark), Gary Remington (Canada), Christian Simhandl (Austria), Rael Strous (Israel), Takefumi Suzuki (Japan), Jari Tiihonen (Finland), JK Trivedi (India), Eduard Vieta (Spain), Miguel Xavier (Portugal), A. Elif Anil Yagcioglu (Turkey), and Nevzat Yuksel (Turkey).