The co-occurrence of posttraumatic stress disorder (PTSD) and substance use disorders is a major public health concern in the United States because of negative physical and mental health consequences and poor treatment outcomes. Decades of cross-sectional epidemiologic studies repeatedly show high prevalence rates of PTSD among substance using subjects (reviewed by Chilcoat and Menard [
1]). Advances have been made in behavioral and psychopharmacologic treatments for comorbid PTSD and substance use disorders; however, these studies demonstrate only modest improvements in outcome and have yet to specify mechanisms that influence outcomes. In the absence of clear, empirically supported treatment guidelines, significant questions remain. 1) Should PTSD symptoms be targeted to improve substance use outcomes? 2) Should substance use symptoms be targeted to improve PTSD outcomes? 3) Or should both sets of symptoms be addressed for clinically meaningful treatment benefits?
A number of conceptual models have hypothesized the causal pathways between PTSD and substance use disorders and the mechanisms responsible for treatment outcome. The predominant models include those that identify PTSD as a risk factor for substance use disorders (i.e., the "self-medication" hypothesis [
2,
3]), substance use disorders as a risk factor for PTSD (i.e., substance use increases the likelihood of developing PTSD following exposure), and those who posit a shared neurobiological vulnerability (
4). Longitudinal studies demonstrate the most support for the self-medication model (
5–
8); however, these studies have not yet accounted for the relationship between PTSD and substance use symptoms in response to interventions (i.e., how do improvements in one disorder affect the other and vice versa?). A recent study by Back and colleagues (
9) examined this question by investigating the temporal course of improvement in PTSD and alcohol dependence symptoms among 94 individuals participating in a 12-week outpatient medication treatment trial. Improvements in PTSD led to improvements in alcohol dependence symptoms while the reciprocal relationship—improvements in alcohol dependence reducing PTSD symptoms—was not demonstrated. These preliminary findings suggest that 1) co-occurring PTSD symptoms may have a strong impact on alcohol dependence treatment outcome and 2) concurrent forms of treatment, specifically those that address PTSD symptoms early in treatment, may be important in optimizing outcomes for patients with comorbid PTSD and alcohol dependence.
The objective of this analysis was to examine the temporal course of improvement in PTSD and related substance use in the context of a clinical trial, addressing the limitations of the extant literature. The current group of women participated in sessions of either trauma-focused or health education group treatment implemented in community-based substance abuse treatment programs within the National Institute on Drug Abuse (NIDA) Clinical Trials Network. Frequent PTSD symptom and substance use measurements were taken longitudinally to facilitate causal modeling. The primary research objectives were 1) to test temporality in the relationship between PTSD and substance use symptom improvements weekly over the course of active study intervention, and 2) to test the relationships between PTSD reductions and substance use symptom outcomes over the 12-month longitudinal follow-up period, comparing those who received trauma-focused with those who received health education group treatment.
Method
Participants
Participants were included on the basis of the following criteria: 1) meeting DSM-IV criteria for full or subthreshold PTSD (subthreshold PTSD requires that participants meet either symptom cluster C or D, instead of both), 2) substance use within the past 6 months and a current diagnosis of drug or alcohol abuse or dependence, 3) age 18–65, and 4) proficiency in English. Exclusion criteria were significant risk of suicidal/homicidal intent or behavior, a history of schizophrenia spectrum diagnosis, or active (past 2 months) psychosis.
Study Design
Seven community-based treatment programs offering intensive outpatient treatment participated in the study. Sites were situated in urban (N=5) and suburban (N=2) settings and were geographically located in the western (n=1), midwestern (n=1), northeastern (n=2), and southeastern (N=3) United States.
Recruitment occurred over 21 months in 2004 and 2005. Written informed consent was obtained from participants after complete description of the study. After completion of an eligibility screening followed by a baseline assessment, participants were randomly assigned to a treatment group. Randomization was stratified by prescription psychotropic medication use and substance use diagnosis (alcohol use disorder only [8.8%] versus drug use disorder only [37.7%] or concurrent drug and alcohol use disorders [53.5%]). Substance use and PTSD symptoms were assessed weekly during treatment, with full assessment repeated posttreatment after 1 week and after 3, 6, and 12 months. Independent assessors, blind to randomized assignment, conducted baseline and posttreatment assessments.
Study Interventions
After randomization, women attended an initial individual session with the counselor to discuss intervention assignment, group format, and rules. Groups had an open, rolling enrollment format, lasted approximately 75–90 minutes, and ran as long as three or more women were enrolled. Because of the criterion of needing two women present to conduct a group, many women took longer than 6 weeks (two group sessions per week) to complete the interventions. All participants continued to attend treatment as usual offered by their treatment programs.
Trauma-focused group treatment.
"Seeking Safety" is a structured cognitive behavior treatment with both safety/trauma and substance use components integrated into each session (
10). Seeking Safety was abbreviated from 25 to 12 core sessions to better fit standard substance abuse treatment duration. Sessions include basic education on substance use disorders and PTSD, skill-building to prevent drug use and manage PTSD symptoms, cognitive restructuring with attention to maladaptive thoughts linked to substance use and trauma symptoms, and a focus on developing effective communication skills to build healthy support networks. All sessions had the same format: 1) check in, including reports of good coping skills or any "unsafe" behaviors; 2) session quotation, a brief point of inspiration to engage participants and link to session topic; 3) relating the material to patients' lives, in which handouts are used to facilitate discussion and skill practice; and 4) check out, including a commitment to specific between-session skills practice. Each session covered a different topic (e.g., "PTSD: Taking Back Your Power" and "When Substances Control You").
Health education group treatment.
The "Women's Health Education" control condition was adapted from a treatment grant protocol for female partners of injection drug users (unpublished 1998 treatment manual of Miller, Padian, and Tross). It is a psychoeducational, manualized treatment focused on topics such as pregnancy, nutrition, diabetes, hypertension, and HIV/sexually transmitted diseases. Women's Health Education was designed to provide equivalent therapeutic attention, expectancy of benefit, and an issue-oriented focus, but without the theory-driven techniques of Seeking Safety or any explicit focus on substance abuse or trauma. Sessions followed a common format, including reviewing between-session assignments, topic presentation with accompanying video or text, group exercises, and goal-setting.
Counselors and supervisors from each treatment program were centrally trained in their respective study interventions and later certified upon successful completion of a training group of at least four sessions. All intervention sessions were videotaped and 50% rated for adherence by supervisors. The lead team randomly selected and rated 25% of tapes reviewed by local supervisors to assure fidelity and interrater reliability.
Assessments
PTSD was measured with the Clinician-Administered PTSD Scale (CAPS [
11]), a structured interview that measures DSM-IV PTSD diagnosis and the frequency and intensity of symptoms over the past 30 days. The total severity score has a range of 0–136. The scale was administered at baseline and at all follow-up timepoints. The lead team conducted diagnostic reliability checks by listening to a subset (20%) of audiotaped scale assessments and held weekly conference calls with independent assessors to maintain competency and discuss challenging clinical issues. The self-report PTSD Symptom Scale (
12) was administered at all assessment timepoints, including the treatment phase, to measure the frequency and intensity of PTSD symptoms (
12).
The Addiction Severity Index-Lite (
13) was used to assess prior 30-day substance use at baseline and follow-up timepoints. The maximum number of days of use across 10 substance use categories (alcohol, cocaine, marijuana, opiates, sedatives, stimulants, heroin, barbiturates, inhalants, and hallucinogens) was categorized into three levels: abstinence (no use), light use (used 1–12 days), and heavy use (used 13 or more days [i.e., more than 3 days per week]) (
14). The Addiction Severity Index alcohol and drug composite scores ranged from 0 to 1 (
15). Each alcohol composite score was recoded into four levels: abstinence, light (0.01–0.15), median (0.16–0.40), and heavy (0.41 or higher); for the drug composite score, the levels were abstinence, light (0.01–0.10), median (0.11–0.20), and heavy (0.21 or higher). The cut points were determined statistically, chosen to equalize the sample in each level among users at baseline. The Substance Use Inventory is a series of self-report questions about quantity and frequency of substance use in the past 7 days adapted from the Timeline Followback measure (
16). The Substance Use Inventory was administered at all assessment time points, including the treatment phase.
Defining Response During the Treatment Phase
Consistent with scoring practices used by Brady and colleagues (
17) for the measurement of clinically significant changes in PTSD symptoms, improvement was defined as a 30% or greater reduction from baseline to each intervention visit. Substance use improvement was defined according to the scoring conventions of Nunes and colleagues (
18) as a 75% or greater reduction of drug/alcohol using days per week, measured by the Substance Use Inventory, compared with baseline levels. At each intervention phase visit, participant improvement was classified into one of four categorical variables: 1) nonresponse: no improvement in either PTSD or substance use severity, 2) substance use response: improvement in substance use symptoms only, 3) PTSD response: improvement in PTSD symptoms only, and 4) global response: improvement in both PTSD and substance use symptoms. A fifth category, dropouts, was used for those who were no longer in treatment.
Statistical Analyses
The first of two analytic methods was applied to investigate the temporality of the association between improvement in PTSD severity measured by the PTSD Symptom Scale and improvement in drug and alcohol use during the 6-week treatment phase of the study. A continuous Markov model was fit on the aforementioned four defined response categories.
The second strategy was applied longitudinally to test the relationship between PTSD and substance use symptom changes over the course of the 1-year follow-up period and involved the application of generalized linear models. The generalized linear model was applied for repeated outcome measures, observed at 1 week, 3 months, 6 months, and 12 months after treatment. The three main outcomes were ordinal measures of 1) the maximum number of days used across 10 substances in the past 30 days, 2) Addiction Severity Index alcohol composite score, and 3) Addiction Severity Index drug composite score. Each of the three outcomes was modeled as a function of PTSD changes from baseline to each assessment point; intervention type (trauma-focused or health education); time of assessment; baseline level of the relevant outcome variable; and preselected baseline covariates (race, age, education, and marital status). The possible interactions between PTSD changes, intervention type, and the baseline level of the outcome measure were tested and included in the final model if significant. In order to examine the difference in treatment effect among programs, site was included as an additional fixed effect, while the participant was a random variable. Generalized estimating equations (
20) were used to estimate and test the models. The generalized estimating equations methodology is able to handle within-subject correlation arising from repeated measurements, requires no parametric distribution assumption for the outcomes, provides robust inference with respect to misspecification of the within-subject correlation, and considers missing at random. PROC GENMOD in SAS (SAS 9.1.3) was used to conduct the analysis.
Results
Participants
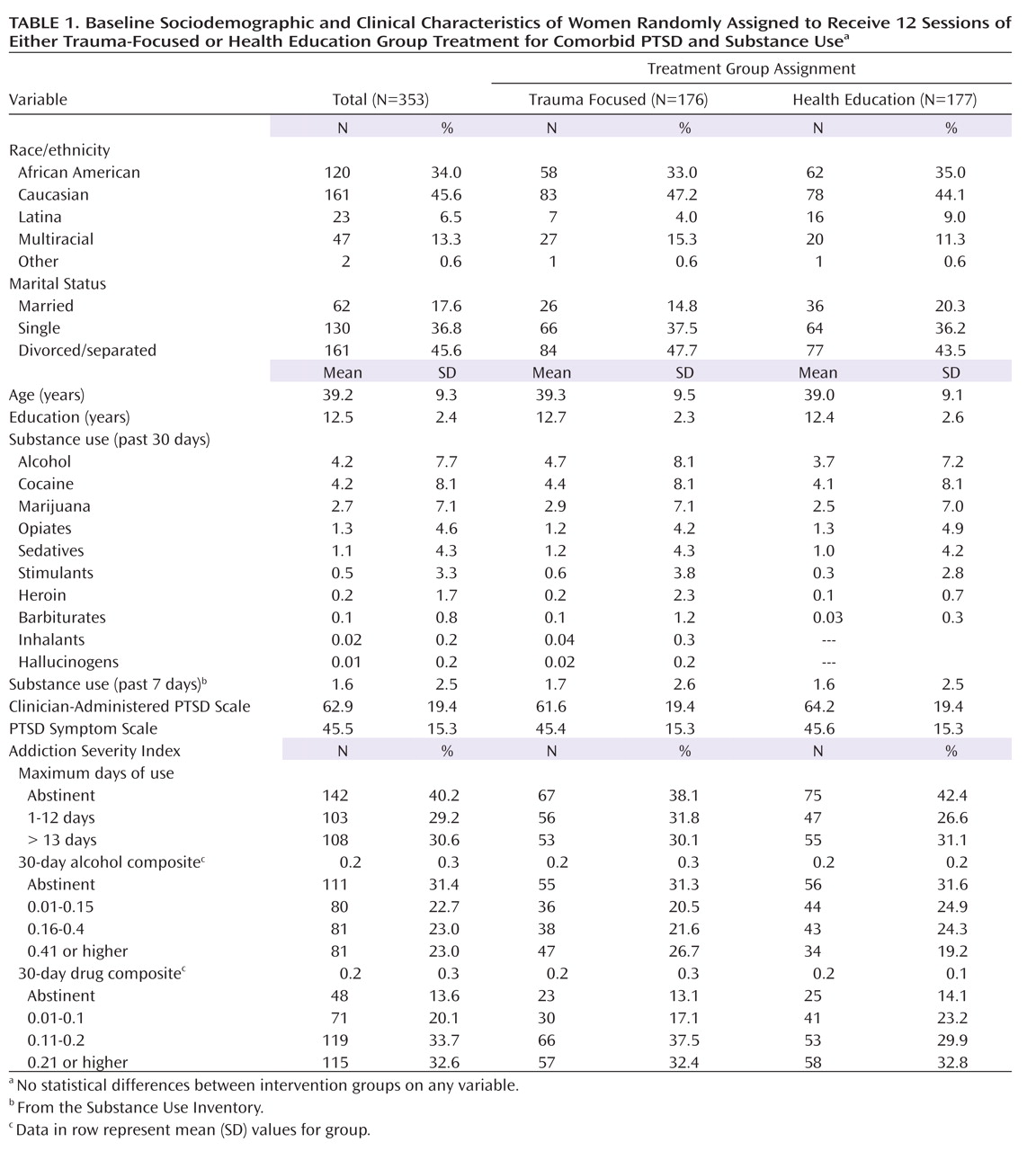
Demographic and clinical characteristics for the 353 women assigned to a treatment group are displayed in
Table 1. There were no significant differences between treatment groups on sociodemographic factors, PTSD symptoms, or drug use at baseline. The average age of the sample was 39.2 years. Forty-six percent were Caucasian, and one-third were African American. About half were divorced or separated, and 37% were never married.
As specified by study eligibility criteria, all participants met current DSM-IV criteria for either full (80.4%) or subthreshold PTSD (19.6%). The average CAPS score (62.9, range=19–119) was in the severe range. The most frequent substances used in the past 30 days were cocaine and alcohol; given that the sample was recruited within substance abuse treatment programs, 40% were abstinent at baseline.
Eighty-two percent of participants attended at least one treatment session, with 56% attending six or more (the a priori definition of treatment completion). Retention rates were similar at each follow-up point (61%–63%) and did not differ significantly by study intervention type or frequency of drug use at baseline. Eighty-two percent of the participants had at least one posttreatment assessment.
Temporal Association Between PTSD and Substance Use Improvement
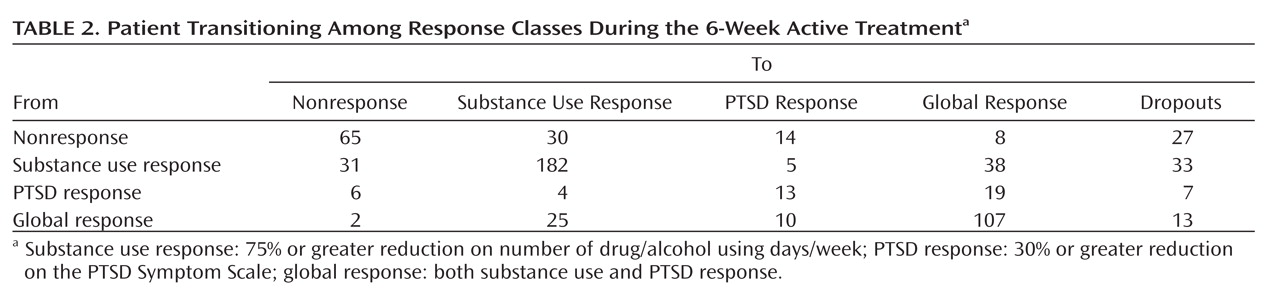
In order to test our first hypothesis that PTSD reductions lead to changes in substance use, we examined data collected weekly during active study treatment. There were 639 transition events modeled, and
Table 2 displays the proportion of transitions to each of the five classifications in successive weeks following baseline. Participants exhibiting nonresponse, substance use response, and global response tended to maintain their original classification (e.g., if they improved in substance use only, they were most likely to remain a substance use responder). Participants who were initially classified as PTSD responders, however, were significantly more likely to transition to global response over time, indicating maintained PTSD improvement was associated with subsequent substance use improvement.
Inferential tests of treatment effects in the Markov model yielded no overall significant treatment effect (χ
2=9.72, df=7, p=0.21) and no significant treatment effects on individual transition intensity. That is, the association between PTSD and substance use did not differ between the two interventions.
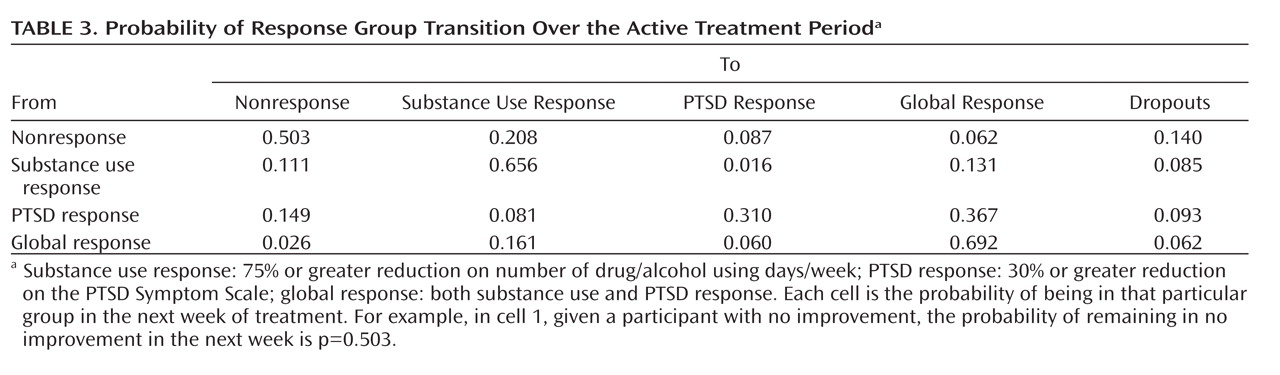
Table 3 displays the estimated transition probability matrix in successive weeks based on model 1. Subjects exhibiting PTSD response were approximately 2.80 (0.37/0.13) times (95% CI=1.52–4.58, based on 1,000 bootstrapping repetitions) more likely (in probability) than those exhibiting substance use response to change to global response within 1 week.
Longitudinal Association Between PTSD Change and Substance Use Outcomes
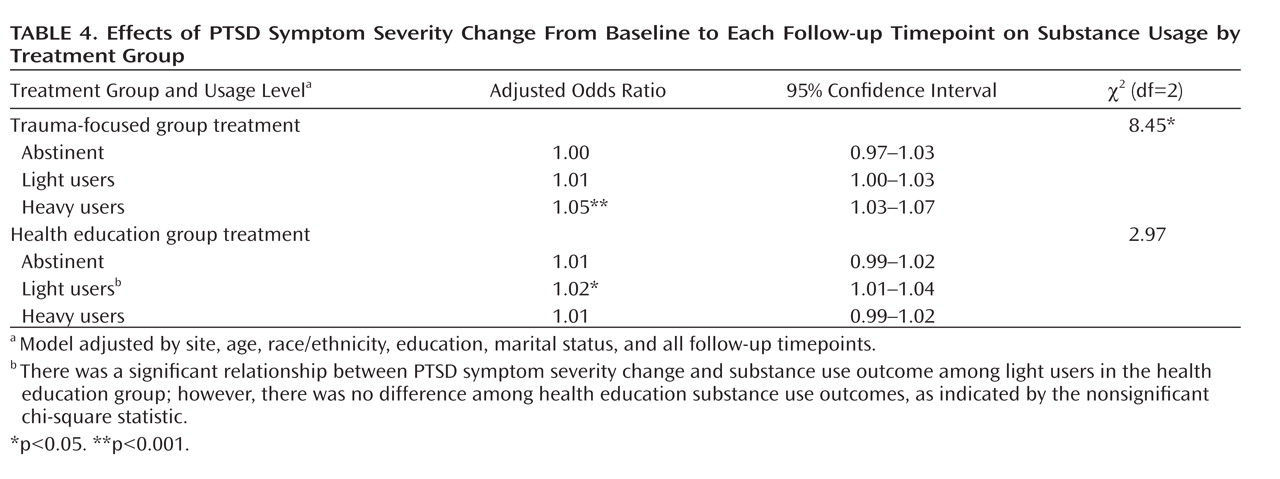
The generalized multinomial logistic models of substance use over the follow-up period (after 1 week and after 3, 6, and 12 months) indicated a significant effect of changes in CAPS total score from baseline to each follow-up point (
Table 4). During the 12-month follow-up, maximum days of use and Addiction Severity Index alcohol and drug composite scores were all significantly related to the improvements in substance use and PTSD symptoms from baseline to each follow-up point. Further, for both maximum days of use and drug composite scores, a three-way interaction between treatment group, baseline level of substance use, and PTSD improvements from baseline to each follow-up point was found such that the impact of PTSD improvement on substance use at follow-up significantly differed by treatment group and baseline level of drug use (χ
2=8.07, df=2, p=0.02 for maximum days of use, χ
2=9.19, df=2, p=0.03 for drug composite). Among women assigned to trauma-focused group treatment, one unit of improvement on CAPS score for those who were heavy substance users at baseline decreased the odds of being in the heavy users group at follow-up by 4.6% (z score=4.35, p<0.001), relative to 1.3% (z score=1.49, p=0.13) for light substance users and no impact for those abstinent at baseline. Among women assigned to health education group treatment, one unit of improvement on CAPS score for those who were heavy substance users at baseline decreased the odds of being in the heavy users group at follow-up by 0.6% (z score=0.75, p=0.45); the corresponding reductions in likelihood for being in the heavy users group at follow-up for each unit of CAPS improvement were 2.3% (z score=2.60, p=0.009) and 0.6% (z score=0.66, p=0.51) for those who were light users and abstinent at baseline, respectively. Among those who were heavy substance users at baseline, the effect of the improvement of scale scores differed significantly by treatment group (z score=2.95, p=0.003); there was no significant treatment group effect for those who were light substance users at baseline (z score=0.79, p=0.43).
The effects on the alcohol composite differed from the two drug use outcomes revealing a two-way interaction effect between CAPS improvement and baseline alcohol use. The effect of one unit of improvement of CAPS severity score on the probability of being a heavy alcohol user was stronger for baseline heavy alcohol users than light users (χ2=15.85, df=3, p=0.001).
Discussion
When the comorbidity between PTSD and substance use disorders during active study intervention was examined, PTSD changes were found to impact substance use outcomes. Specifically, PTSD severity reductions were associated with substance use disorder improvement, with minimal evidence of substance use reduction improving PTSD symptoms. The findings, derived from two different sets of analyses spanning week-to-week probability data and longitudinal follow-up data, support the self-medication model as applied to populations with comorbid PTSD and addictive disorders.
Moreover, as predicted, PTSD-targeted treatment (Seeking Safety) was significantly more effective in achieving substance use improvement than the comparison treatment, but only among those with heavy baseline substance use who had achieved significant PTSD reductions. Indeed, Seeking Safety is an integrated cognitive behavior approach that actively links PTSD symptoms with "unsafe" substance use behaviors and whose efficacy has been largely demonstrated with active substance users (
21). Accordingly, those in the sample who were abstinent at baseline may have benefited less from a focus on PTSD symptoms as related to substance use behaviors relative to those with active substance use. We further speculate that Seeking Safety was superior to the control condition for this group because those with more substance use also had more severe PTSD. In fact, baseline PTSD scores were statistically different among three levels of substance use defined by the maximum number of days of use (p<0.05), such that those with heavy substance use at baseline had more severe PTSD. If substances are used to self-treat PTSD symptoms, then daily substance use may be considered a proxy for greater PTSD severity. This finding suggests that while for the average dually diagnosed patient the additional benefits of PTSD-targeted interventions may be limited, PTSD-focused treatments like Seeking Safety offer an advantage to patients with more severe PTSD and substance use symptoms.
Although only the subset of study participants with more severe baseline substance use appeared to benefit from the specific elements of Seeking Safety, this finding is in line with other treatment studies that show the largest treatment effects among those with the most severe problems (
22). Because the detection of mediation in clinical trials is inherently a low-power endeavor, it would be expected that differences would be most clearly observed in the subset of the sample where the intervention effect is most powerful, namely those with a high level of the baseline treatment targets (i.e., higher levels of substance use).
Overall, our results have important clinical implications for treating women with comorbid PTSD and substance use disorders. The findings further contradict conventional wisdom that addressing trauma-related symptoms will negatively impact substance use recovery. Instead, we demonstrate that trauma-focused treatment can lead to improvements in substance use outcomes in the context of PTSD symptom reductions, without decreasing participant attendance. Thus, we contend that the most effective treatment models are those that address PTSD before substance use or simultaneously. We propose this course of treatment, in contrast to treatment commonly offered in substance abuse treatment settings that lack a trauma-focus, especially because of the high prevalence rates of trauma histories and PTSD among such patients.
Our study has several limitations. First, 40% of the sample was abstinent at baseline, which restricted the variability in alcohol and drug outcomes and thereby could have diluted the overall treatment effect. This is particularly true with respect to alcohol outcomes, as the vast majority of the sample met drug abuse or dependence criteria, with or without concurrent alcohol abuse or dependence (91.2%). Therefore, the findings may not generalize to a primarily alcohol-dependent sample. Second, the cohort consisted entirely of women, which precludes extrapolation of results to men. A third consideration is that the participants received study interventions while enrolled in substance abuse treatment; receiving additional treatment focused on managing addictive behavior may have influenced outcomes.
The present study is only the second attempt of which we are aware to examine and test the temporal course of PTSD and substance use disorder symptom change. These data afforded a unique opportunity to discriminate between different mechanisms of comorbidity due to repeated and longitudinal measurement of symptoms. Our results offer support for the self-medication model and an empirical basis for PTSD-focused and integrated interventions for improved substance use outcomes in patients with severe symptoms. Future studies should investigate these issues with men and examine the efficacy of PTSD-focused treatments for patients with varied substance use patterns to determine if such treatments are superior for them as well.
Acknowledgments
The authors would like to acknowledge the Clinical Coordinating Center staff at the Clinical Trials Network, who collaborated in the design and conduct of the study and assisted with data management, quality assurance during the trial, and comments for consideration on the final draft of the manuscript. The authors also acknowledge the work of the research and clinical staff at the seven participating community substance abuse treatment programs and the women who participated in this clinical trial.