Several recent neuroimaging studies have reported functional abnormalities in the anterior cingulate cortex in posttraumatic stress disorder (PTSD). The anterior cingulate is a structure in the medial prefrontal cortex that comprises several functional subdivisions (
1,
2). Rostral regions of the anterior cingulate activate during emotional states and during tasks that involve interference from emotional stimuli (
3–
8). In contrast, dorsal regions of the anterior cingulate (
2,
9) typically activate during a wide variety of tasks that involve interference from nonemotional stimuli (
5). The dorsal anterior cingulate is thought to play a role in multiple cognitive processes, such as performance monitoring, response selection, error detection, and decision making (
5,
6,
10,
11).
In PTSD, rostral portions of the anterior cingulate appear to be hyporesponsive during the presentation of trauma-related and other negative stimuli (
12–
18) and during emotional interference tasks (
19,
20). In contrast, dorsal portions appear to show exaggerated responsivity in PTSD during Stroop interference, oddball tasks, fear conditioning, and extinction recall (
12,
20–
25), although not all studies have reported this finding (
26–
29).
Whether functional abnormalities in the anterior cingulate in PTSD are acquired characteristics of the disorder or are familial risk factors for the development of PTSD after trauma is unclear. In order to begin to address this question, we recently used positron emission tomography (PET) and fluorodeoxyglucose (FDG) to study resting regional cerebral metabolic rate for glucose in Vietnam combat veterans with and without PTSD, as well as in their combat-unexposed identical co-twins without PTSD (
30). We found that resting metabolic rate in a region that included the dorsal anterior cingulate and midcingulate cortex was elevated in the combat veterans with PTSD and their combat-unexposed co-twins without PTSD, as compared to combat veterans without PTSD and their co-twins. In addition, we found a significant positive correlation between resting metabolic rate in this region in the unexposed twins and their exposed twins' PTSD symptom severity scores. Overall, these findings suggest that resting hypermetabolism in the dorsal anterior cingulate is a familial risk factor for the development of PTSD after psychological trauma.
Interpreting the cognitive correlates of metabolic rate abnormalities in a given structure at rest is challenging because the finding is, by definition, unassociated with a specific cognitive task. To help clarify the role of the dorsal anterior cingulate as a possible risk factor for the development of PTSD, we examined its function using functional MRI (fMRI) and the Multi-Source Interference Task, which yields behavioral indicators of cognitive interference and reliably activates the dorsal anterior cingulate in healthy individuals (
9,
31). We studied Vietnam combat veterans with and without PTSD, as well as their combat-unexposed identical co-twins. According to the logic of the design (
32), a finding of exaggerated dorsal anterior cingulate activation both in the combat veterans with PTSD and in their identical co-twins would be consistent with a familial risk factor. In contrast, exaggerated dorsal anterior cingulate activation in the combat veterans with PTSD and not in their identical co-twins would be consistent with an acquired characteristic of PTSD. Based on previous PET-FDG findings, we hypothesized that combat veterans with PTSD and their identical co-twins would show greater activation in the dorsal anterior cingulate as compared to veterans without PTSD and their identical co-twins. Given minimal previous evidence of significantly elevated response times or error rates on nonemotional Stroop interference tasks in PTSD (
19,
33–
35), we had no directional a priori hypotheses regarding group differences on these behavioral measures.
Method
Participants
Participants were drawn from a pool of male identical twins who had participated in a previous study (
36). Twenty-six pairs participated; 18 of these pairs participated in our previous PET-FDG study (
30). Each combat-exposed twin had served in the Vietnam combat theater, whereas his combat-unexposed co-twin had not. Of the exposed twins, 12 had developed current combat-related PTSD, and 14 had never developed PTSD, as determined by the Clinician-Administered PTSD Scale (CAPS;
37) using criteria from DSM-IV. Thus, there were four participant groups: combat-exposed veterans who had current combat-related PTSD (N=12) and their combat-unexposed co-twins (N=12), and combat-exposed veterans who never had combat-related PTSD (N=14) and their combat-unexposed co-twins (N=14). The study was approved by the Partners HealthCare System Institutional Review Board. All participants provided written informed consent.
Demographic and Clinical Characteristics
Forty-nine participants were right-handed, and three (one combat-exposed with PTSD; two combat-exposed without PTSD) were left-handed. None of the participants reported a history of major head injury involving loss of consciousness for more than 10 minutes, tumor, epilepsy, cerebrovascular accident, or other neurological disorder.
According to the Structured Clinical Interview for DSM-IV (
38), participants in the combat-exposed group with PTSD met criteria for current comorbid diagnoses: major depression (N=4), dysthymia (N=2), panic disorder (N=3), social phobia (N=1), specific phobia (N=2), and cannabis dependence (N=1). Combat-unexposed co-twins of the PTSD group met criteria for the following current diagnoses: major depression (N=1), bipolar disorder (N=1), specific phobia (N=3), panic disorder (N=1), alcohol dependence (N=1), and civilian PTSD (N=1). Analyses were conducted both with and without the participant with civilian PTSD and his co-twin. Among combat-exposed veterans who never had PTSD, current disorders included dysthymia (N=1) and specific phobia (N=1), and among their combat-unexposed co-twins, two met criteria for current dysthymia.
Eight participants were taking antidepressants at the time of study: three combat-exposed participants with PTSD and one unexposed co-twin, and one combat-exposed participant without PTSD and three unexposed co-twins. Six participants were taking other types of medications: in the combat-exposed group with PTSD, two were taking opioids, one a benzodiazepine, one an antipsychotic, and one a sympatholytic. One co-twin of a combat-exposed participant without PTSD was taking a sympatholytic.
Participants completed the Beck Depression Inventory (BDI;
39), the Michigan Alcoholism Screening Test (MAST;
40), the Childhood Trauma Questionnaire (
41), and a measure of the severity of combat exposure (
42).
Task Procedures
In the Multi-Source Interference Task, participants viewed sets of three numbers or letters (possible numbers were 1, 2, and 3; letters were always x) on a computer screen projected via a tilted mirror. They were told that one number would always be different from the other two items, which would match one another. Participants were asked to report via button-press the identity of the number that was different from the others; on the button-press device, the three buttons represented the numbers 1, 2, and 3, respectively. Participants were also instructed to respond as quickly as possible while minimizing errors and to complete 48 practice trials before beginning the experiment.
The task consisted of two conditions presented in separate alternating blocks. In the control (C) condition, the identity of the target number always matched its position on the button-press device. In the interference (I) condition, the identity of the target number never matched its button-press position (sample stimuli are shown in the data supplement that accompanies the online edition of this article). The task began and ended with 30 seconds of fixation (F), which consisted of a white dot presented in the middle of the screen.
Stimuli were presented with MacStim 3, a stimulus presentation software package, on a Macintosh Powerbook computer. Each stimulus remained on the screen for 1.5 seconds with 0.25 seconds between stimuli. Each block consisted of 24 stimuli. Each run consisted of 10 blocks of alternating conditions in a fixed order (FCICICICIF) and lasted a total of 6 minutes, 36 seconds. Participants completed up to three runs of the Multi-Source Interference Task. Because response time differences between conditions (I – C) and activation of the dorsal anterior cingulate can decline with each additional run in this task, we decided a priori to analyze data from the first run only (
9). (Results of the second run are provided in the online data supplement.)
fMRI Procedures
We used a Symphony/Sonata 1.5-T high-speed scanner (Siemens Medical Systems, Iselin, N.J.) with a three-axis gradient head coil. After shimming, high-resolution structural MRI images (three-dimensional magnetization-prepared rapid acquisition with gradient echo; repetition time=2.73 sec, echo time=3.31 msec, flip angle=7°) with a 1.3-mm slice thickness were collected. fMRI blood-oxygen-level-dependent (BOLD) images were acquired using a gradient echo T2*-weighted sequence (repetition time=1.5 sec, echo time=40 msec, flip angle=90°) in 16 coronal slices perpendicular to the anterior commissure-posterior commissure line (thickness=5 mm, 1 mm skip).
Data Analysis
Behavioral analyses.
Response times from only correct trials were averaged within each condition and run for each participant. Errors were expressed for each participant as a percentage of the total number of trials on which the participant responded within each condition and run. Difference scores were created by subtracting the average response time in the control condition from the average response time in the interference condition. Difference scores were likewise calculated for error rates.
fMRI analyses.
We conducted two types of analyses on the fMRI data: whole-brain voxelwise comparisons and then analysis of variance (ANOVA) of fMRI data that were extracted from the dorsal anterior cingulate in the voxelwise maps of individual participants. In both types of analyses, we treated exposed versus unexposed co-twins as a repeated measure (i.e., main effect of exposure). In addition, we treated the twin pairs in which the combat-exposed twin had PTSD as a separate group from the twin pairs in which the exposed twin never had PTSD. A significant difference between these two groups (i.e., a significant main effect of PTSD diagnosis) would be consistent with a familial risk factor (as long as there was also no interaction between PTSD diagnosis and exposure). This finding would indicate that the combat-exposed twins with PTSD have the same functional abnormality as their unexposed co-twins without PTSD. A significant PTSD diagnosis-by-exposure interaction that reflected an abnormality in only the exposed twins with PTSD would indicate an acquired sign of PTSD. Lastly, a significant main effect of exposure (i.e., a significant difference between all combat-exposed twins as compared to all combat-unexposed twins collapsing across PTSD diagnosis) in the absence of an interaction would suggest that the functional abnormality is associated with exposure to combat and not with PTSD.
Voxelwise analyses.
Statistical parametric mapping analysis of the imaging data was conducted using the SPM2 software package (
www.fil.ion.ucl.ac.uk/spm/software/spm2). Each participant's functional images were motion corrected and coregistered to his high-resolution structural MRI image. The resulting images were spatially normalized in a standard stereotactic space (Montreal Neurological Institute, MNI) and then smoothed (8 mm full width at half maximum). At each voxel, the BOLD data were fitted to a linear statistical model by the method of least squares. Hypotheses were tested as contrasts in which linear compounds of the model parameters were evaluated using t statistics, which were then transformed to z scores.
We used an approach that consisted of two hierarchical levels of analysis, in which the second level's random-effects analysis absorbed the random effects from the first level. First, interference-versus-control contrast images were generated for each participant. For the purpose of examining the main effect of PTSD diagnosis, the contrast images of the combat-exposed and combat-unexposed participants were averaged (first level), and then the PTSD and non-PTSD pairs were compared (second level). For the purpose of examining the PTSD diagnosis-by-exposure interaction, the contrast images of the combat-exposed and combat-unexposed participants were contrasted (first level), and then the PTSD and non-PTSD pairs were compared (second level). Then, for the purpose of examining the main effect of combat exposure, the contrast images of the combat-exposed and combat-unexposed participants were compared in a two-group t test.
The statistical parametric maps resulting from the above analyses were inspected for main effects and their interaction in the dorsal anterior cingulate, which was defined as the portion of the anterior cingulate that is superior to the corpus callosum, between y=0 and y=+30 mm (
43). Given our strong a priori hypotheses, we applied a significance threshold of 0.001, one-tailed and uncorrected (z score ≥3.09), to activations in the dorsal anterior cingulate. For regions about which we had no a priori prediction, we applied a more conservative constant significance threshold of 0.00002, two-tailed and uncorrected (z score ≥4.27) (
29,
30).
Region-of-interest analyses.
We extracted fMRI data from the dorsal anterior cingulate activation at the maximum voxel within each participant, using previously established methods (
9). (Data could not be extracted for four participants [two combat-exposed twins with PTSD, one combat-exposed twin without PTSD, and one of their co-twins] because the maximum voxel value of their interference-versus-control activations did not fall within the dorsal anterior cingulate proper.) We then further analyzed these data for the main effects of PTSD diagnosis and combat exposure and their interaction using a mixed model that treated combat exposure as a within-pairs repeated measure, PTSD diagnosis as a between-pairs measure, and twin pairs as a random effect (
44). We also performed correlational analyses with the extracted data in order to determine whether dorsal anterior cingulate activation in all combat-exposed twins was correlated with their own CAPS scores, as well as whether dorsal anterior cingulate activation in the combat-unexposed twins was correlated with their combat-exposed twins' CAPS scores and other clinical measures. All p values are two-tailed except as otherwise indicated.
Results
Table 1 summarizes the demographic and clinical characteristics of the study participants.
Behavioral Results
Response time and error rate difference scores (I–C) were submitted to separate 2×2 (PTSD diagnosis by exposure) repeated-measures ANOVAs. Regarding response time difference scores, a main effect of PTSD diagnosis was detected but fell short of statistical significance (F=3.61, df=1, 24, p=0.07). The combat-exposed participants with PTSD and their co-twins tended to have larger response time difference scores than the combat-exposed participants without PTSD and their co-twins (
Table 2). No other effect was significant. When we excluded from the analysis the PTSD twin pair in which the unexposed co-twin had civilian PTSD, the main effect of PTSD diagnosis became significant (F=5.30, df=1, 23, p=0.03).
Regarding error rate difference scores, no effects were significant, even when the PTSD pair in which the unexposed co-twin had civilian PTSD was excluded from the analyses.
fMRI Results
Voxelwise analyses.
There was a significant main effect of PTSD diagnosis in the dorsal anterior cingulate (MNI coordinates, x=+10, y=+6, z=+46; z score=3.17; k at p<0.001=1). Combat-exposed veterans with PTSD and their co-twins exhibited greater BOLD signal changes in the interference-versus-control contrast than combat-exposed veterans without PTSD and their co-twins. When we ran a small-volume correction based on the volume of the right dorsal anterior cingulate in this sample, the false discovery rate p value for the main effect of PTSD diagnosis was 0.05. The small-volume correction based on the volume of the left and right dorsal anterior cingulate yielded a false discovery rate p value of 0.08. Contrasts between the subgroups revealed a similar pattern (
Table 3).
No regions exhibited significantly lower BOLD signal changes in the PTSD twin pairs relative to the non-PTSD twin pairs. No brain regions met significance thresholds for a main effect of exposure or a diagnosis-by-exposure interaction.
Region-of-interest analyses.
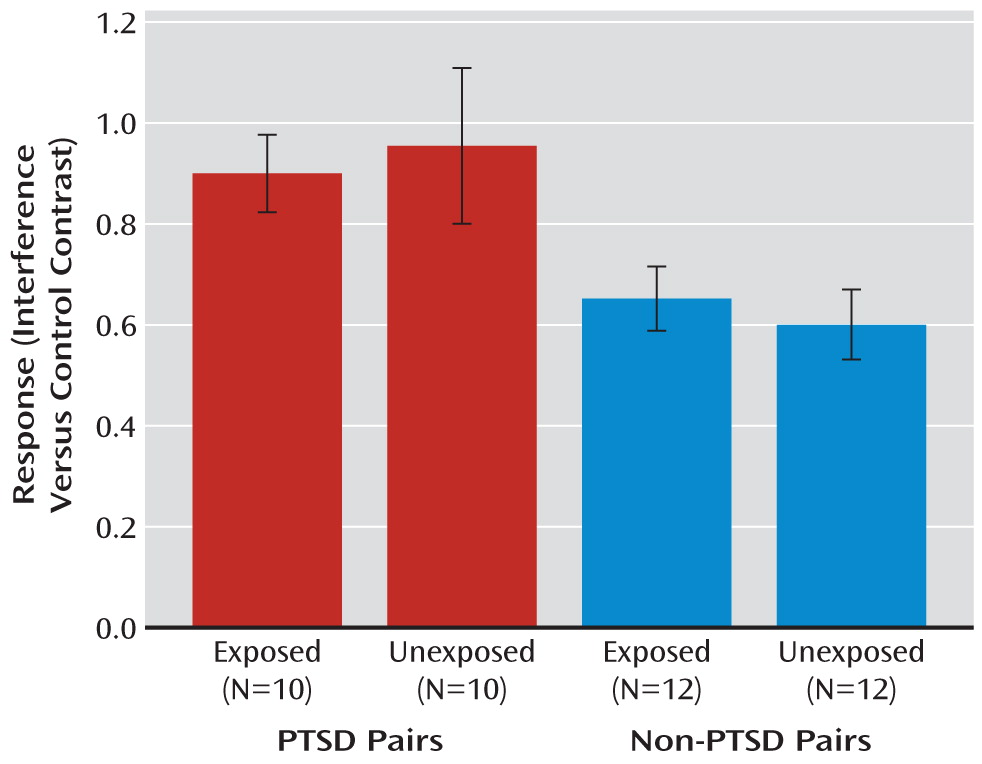
Data from the dorsal anterior cingulate activation in individual participants were extracted and further analyzed. The main effect of PTSD diagnosis was significant (F=8.9, df=1, 20, p=0.007;
Figure 1) and remained significant after exclusion of the PTSD pair in which the combat-unexposed co-twin had civilian PTSD (F=7.9, df=1, 19, p=0.01).
The following covariates were tested as potential confounders of the main effect of PTSD diagnosis by examining their association with the dependent measure using a screening threshold of p<0.20: birth weight, age, total score on the Childhood Trauma Questionnaire, years of education, BDI score, MAST score, diagnosis of major depression, use of psychiatric medication, left-handedness, and severity of combat exposure. Only age met this threshold. Adjusted for age, the main effect of PTSD diagnosis was significant (F=4.9, df=1, 19, p=0.04).
BOLD signal changes in the dorsal anterior cingulate in the combat-exposed twins were positively correlated with their own current CAPS scores (r=0.37, N=21, p=0.043, one-tailed; see the online data supplement). BOLD signal changes in the dorsal anterior cingulate in the combat-unexposed twins were positively correlated with their combat-exposed twins' current CAPS scores (r=0.34, N=23, p=0.05, one-tailed; see the online data supplement) and combat exposure scores (r=0.38, N=22, p=0.03, one-tailed). However, these correlations became nonsignificant when a potential outlier was removed. BOLD signal changes in the dorsal anterior cingulate in the combat-unexposed twins were not significantly correlated with their combat-exposed twins' BDI scores or response time difference scores. In a post hoc analysis, response time difference scores in the combat-unexposed twins were not significantly correlated with their combat-exposed twins' CAPS scores.
Discussion
Vietnam combat veterans with PTSD and their identical co-twins showed greater fMRI activation in the dorsal anterior cingulate during a nonemotional interference task as compared to Vietnam combat veterans without PTSD and their identical co-twins. In addition, activation of the dorsal anterior cingulate in the combat-exposed twins was positively correlated with their own CAPS scores. Furthermore, dorsal anterior cingulate activation in the combat-unexposed twins was positively correlated with their combat-exposed twins' CAPS and combat exposure scores but not with other clinical measures. These results suggest that dorsal anterior cingulate hyperresponsivity is a familial risk factor for PTSD rather than an acquired characteristic of PTSD.
The finding of exaggerated activation in the dorsal anterior cingulate in PTSD is consistent with some previous findings in PTSD singletons (
12,
20–
22,
24,
25), but not all (
26–
29). Two previous studies found exaggerated dorsal anterior cingulate activation during interference tasks in combat veterans with PTSD (
20,
25). In those studies, the exaggerated activation in PTSD was not accompanied by a significant behavioral effect—that is, the response time difference scores were not significantly greater in PTSD than in the trauma-exposed comparison group. This could be attributable to the small sample sizes in those studies. In the present study, with a larger sample size, the behavioral effect approached statistical significance (p=0.07) with all participants included and became significant (p=0.03) when we excluded from the analysis a PTSD pair in which the combat-unexposed co-twin had civilian PTSD. Interestingly, although dorsal anterior cingulate activation in the combat-unexposed twins was correlated with their combat-exposed co-twins' CAPS scores, response time difference scores in the combat-unexposed twins were not. This suggests that dorsal anterior cingulate activation might be a more sensitive measure of vulnerability than behavioral measures of interference.
Our findings in this study are consistent with those of our previous PET-FDG twin study (which included 18 of the same pairs studied here) (
30). Thus, PTSD twin pairs exhibited increased metabolic rates in the dorsal anterior cingulate at rest, as well as greater activation during an interference task involving nonemotional information. Moreover, the locations of these two findings are similar (MNI coordinates in the PET-FDG study: x=+10, y=+2, z=+42; in the present fMRI study: x=+10, y=+6, z=+46). Although these findings await replication in a new sample, it appears that the functional abnormality in the dorsal anterior cingulate in PTSD can be elicited using either technique. With fMRI, radioisotopes are not required, and the implementation of a specific cognitive task helps to pinpoint the cognitive correlates of the functional abnormality.
Exaggerated dorsal anterior cingulate activation in the PTSD group and their identical co-twins could reflect increased cognitive interference and/or response selection, an interpretation that is consistent with the trend for greater response time differences in those groups. This exaggerated activation is not likely attributable to error monitoring as all groups had similar error rate difference scores. Because the task was presented in blocks, we were unable to remove fMRI data from individual trials on which errors occurred. Exaggerated dorsal anterior cingulate activation could also reflect autonomic arousal or its regulation during task performance (
22,
45,
46), although we do not have psychophysiologic measures to support this possibility. However, previous behavioral studies have found that individuals with PTSD are not more physiologically responsive during Stroop interference tasks or other cognitive stressors as compared to control groups (
19,
33,
47). Finally, given that the dorsal anterior cingulate is activated during the expression of conditioned fear responses in healthy humans (
46) and is hyperresponsive during extinction recall in PTSD (
23), the exaggerated activation observed in PTSD in the present study may reflect a general hyperresponsivity of this brain region that could be related to the exaggerated fear responses observed in PTSD. The positive correlation observed between dorsal anterior cingulate activation and PTSD symptom severity is consistent with this speculation.
All participants in this study were male, which may limit the generalizability of the findings. Other limitations include the presence of disorders other than PTSD and medication use in some of the twins. Another possible limitation is the presence of civilian PTSD in one co-twin. However, civilian PTSD in this co-twin did not drive the main effect of diagnosis because the hyperactivation remained even when this twin pair was excluded from the analysis. Given that we did not include combat-exposed individuals who had past or partial PTSD, our findings may not generalize to those conditions. Future studies might implement event-related designs and psychophysiologic monitoring to further clarify the role of dorsal anterior cingulate function as a potential familial risk factor for the development of PTSD following psychological trauma. In addition, future studies should seek to determine whether hyperresponsivity of the dorsal anterior cingulate during interference tasks in PTSD is associated with specific genotypes, such as the presence of a short allele of the serotonin transporter polymorphism, which has been associated with an increased risk of PTSD after exposure to traumatic events (
48).
Acknowledgments
The authors thank Mary Foley and Lawrence White for technical assistance.