Major depressive disorder is prevalent in youths and leads to significant social and academic impairment, increased risk of suicide and substance abuse, and long-term difficulties extending into adulthood (
1). MRI is a noninvasive and safe tool for studying brain function in the pediatric populations and can contribute to our understanding of the impact of depression and its treatment on the developing brain, which is especially important given the dynamic nature of brain growth during youth.
Adult neuroimaging studies have convincingly shown that depression in adults is associated with heightened neural activity in ventral limbic and paralimbic brain regions. Depressed adults show increased regional activity in the amygdala, anterior cingulate cortex, and orbitofrontal cortex compared with unaffected comparison subjects (
2–
6). In addition, depressed adults show reduced activity in dorsal frontal regions, specifically demonstrating decreased dorsal anterior cingulate cortex metabolism and dorsal frontal blood flow relative to healthy comparison subjects (
6,
7).
Functional MRI (fMRI) studies have been underutilized in pediatric depression, and in those that have been conducted, findings have not always been consistent (
8–
13). In addition, studies have been underpowered, with most using small sample sizes. Depression studies that have used a facial expression task paradigm report amygdala hyperactivation in depressed relative to healthy youths (
8,
10,
13). However, amygdala hyperactivation was not evident in two studies that used passive viewing of faces (
8,
11). One study that compared orbitofrontal cortex activations between depressed and healthy youths found no differences under active or passive face viewing conditions (
8). Another study found hyperactivation in depressed youths relative to healthy comparison subjects in the subgenual anterior cingulate cortex during performance of a go/no-go task (
12). Limbic hyperactivation in depressed adults with passive viewing may result from comorbid anxiety or may represent potential differences between adult and pediatric depression (
8,
11).
We have even less knowledge about the impact of antidepressants on the developing brain, even though these medications might affect critical brain regions and circuits in children, with long-lasting effects. The available MRI data are insufficient to compare treatment-sensitive areas in youth and adult populations. Adult studies confirm that antidepressant treatment not only reduces depression symptoms and behaviors but also leads to normalization of neural activation changes within subcortical and limbic brain areas, particularly in the amygdala (
14–
17). To our knowledge, there have been no fMRI studies examining changes in brain activity with treatment in pediatric depression.
In this study, we sought to determine whether the brain regions implicated in pediatric depression include the same regions as those reported in adult depression and whether the fMRI effects of antidepressant treatment in depressed adolescents are similar to those seen in depressed adults. Using voxel-wise whole brain analysis, we examined differences in fMRI activation to emotional faces before and after antidepressant treatment and hypothesized that treatment would be associated with normalization of activation in the amygdala, the orbitofrontal cortex, and the subgenual anterior cingulate cortex.
Method
The study was reviewed and approved by the University of Texas Southwestern Medical Center Institutional Review Board. Written informed consent and assent were obtained from legal guardians and adolescents before the initiation of study procedures, in compliance with the regulations of the Institutional Review Board.
Participants
Twenty-three depressed adolescents (ages 11–17 years) were recruited for the study. While the majority (N=17) were recruited from the outpatient service, several (N=6) were recruited from ongoing treatment studies at the University of Texas Southwestern Medical Center/Children's Medical Center of Dallas. Depressed participants had a history of at least 4 weeks of nonpsychotic major depressive disorder based on DSM-IV criteria, with a score ≥4 on the Clinical Global Impressions severity subscale (CGI-S) (
18) and a total score ≥40 on the Children's Depression Rating Scale–Revised (
19). Patients with concurrent psychiatric disorders were allowed to participate as long as major depression was the primary disorder. Patients were excluded if they had a lifetime history of psychotic depression or bipolar disorders; substance abuse or dependence within the past 6 months; or treatment with psychotropic medications. Three patients withdrew consent before the first scan was conducted, and the imaging data for one participant were discarded because of excessive movement. Thus, analyzable baseline MRI data were available for 19 depressed adolescents. Of these, usable week 8 scans were available for 15 (three were not scanned at week 8, either because of worsening of depression or hospitalization [N=2] or because antidepressant treatment had been discontinued [N=1], and one scan was discarded because of excessive movement).
A total of 22 healthy adolescents (ages 11 to 18 years) were recruited from the community as comparison subjects; none had any current psychiatric illness, lifetime history of psychiatric illness, history of psychotropic medication use, or first-degree family history of psychiatric illness. The imaging data from one comparison subject were discarded because of excessive movement. Thus, usable baseline imaging data were available for 21 healthy comparison subjects. Of these, usable week 8 scans were available for 17 (two participants could not be scanned because they got braces, and one was no longer available after leaving for college; one scan was discarded because of excessive movement).
Procedures and Measures
Adolescents recruited from the depression treatment studies were evaluated by the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (
20), and diagnoses were confirmed by a child psychiatrist. Clinical patients were diagnosed through clinical interviews and a checklist of DSM-IV's nine symptoms of major depressive disorder. Severity of depression was assessed by a child psychiatrist using the Children's Depression Rating Scale–Revised at baseline and at exit. Response to treatment was defined as a reduction of at least 50% on the total score on the Children's Depression Rating Scale–Revised at week 8 relative to baseline. The baseline scans were done within a week after evaluation. After the baseline scan, depressed adolescents started fluoxetine treatment as part of the treatment study protocol or as prescribed by their treating child psychiatrist (R.T. or G.J.E.). Treatment options were discussed with all participants before treatment was initiated. Fluoxetine was started at 10 mg/day, increased to 20 mg/day at week 2, and increased to 30–40 mg by week 8 as clinically indicated. Nonspecific psychotherapies (not cognitive-behavioral therapy or interpersonal therapy) were allowed to continue during fluoxetine treatment (two participants were in supportive therapy), although initiation of new therapy was not permitted during the study. All depressed participants were followed by a child psychiatrist in office visits (six visits for depression study patients and three visits for clinic patients) and telephone contact during the entire study. Depressed adolescents underwent scanning again 8 weeks after the initiation of fluoxetine treatment. Healthy comparison subjects were interviewed and scanned twice, 8 weeks apart.
fMRI
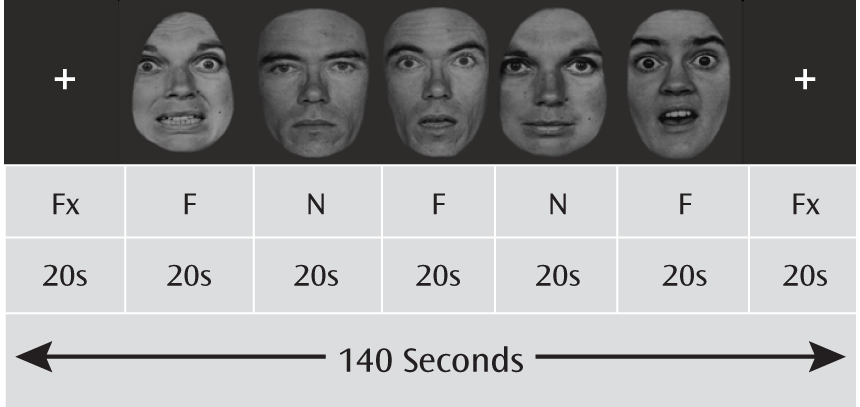
Paradigm and experimental stimuli.
Fearful and neutral facial expressions in five men and five women were selected from the Pictures of Facial Affect collection (
21) and digitized into black and white. The E-Prime software package (Psychology Software Tools, Inc., Sharpsburg, Pa.) was used to present the facial stimuli in a block design, with a fixation block at the beginning and end of each run and five alternating fearful and neutral blocks in between. Each face block presented 10 faces in a randomized sequence, with a 1,500-msec presentation time and a 500-msec interstimulus interval. Each fMRI session included four runs (
Figure 1). A gender discrimination task was used to ensure that participants remained alert during the scan and to minimize cognitive efforts.
fMRI data acquisition.
Blood-oxygen-level-dependent (BOLD) fMRI acquisition was carried out at baseline and at week 8 using a 3-T MR imaging system (Philips Medical Systems, Best, the Netherlands). After a survey scan, T1-weighted high-resolution anatomic images were obtained using a magnetization-prepared rapid gradient echo sequence with an isotropic resolution of 1 mm (duration, 3 minutes 57 seconds). During the functional scans (viewing facial stimuli), a T2*-weighted echo planar imaging sequence was used to acquire BOLD images. Thirty-eight axial slices covering the entire brain were acquired with a repetition time of 2,000 msec, echo time of 30 msec, and voxel size of 2.75×2.75×4 mm (30.25 mm3).
Data Analysis
The SPM5 software package (London, Wellcome Department of Imaging Neuroscience) was used to analyze the functional imaging data. Data were slice-time corrected for an interleaved bottom-up acquisition, adjusted for motion, and spatially normalized into a standard Montreal Neurological Institute (MNI) template. The image volume was then spatially smoothed with a 6-mm full width at half maximum Gaussian filter. Signal changes were modeled using the stimulation paradigm convolved with a canonical hemodynamic response function. Initial single-subject level analyses using a within-subject fixed-effects model were conducted to generate parametric maps for the three block conditions: neutral, fearful, and fixation. Results from the within-subject analyses were then used for subsequent second-level random-effects models.
Whole brain level t tests (two-sample and paired-sample) for the fearful > neutral contrast were conducted initially to guide subsequent region-of-interest analyses. Then a priori region-of-interest analyses were performed in regions of the amygdala, orbitofrontal cortex, and subgenual anterior cingulate cortex. Anatomic masks for the regions of interest were created using the automated anatomical labeling atlas (
22) in the Wake Forest University PickAtlas utility (
23). Activations within the regions of interest had to survive a small-volume correction at a threshold of p<0.05. Single-subject percent signal changes were then calculated using the MarsBaR toolbox in SPM5 (
24). Functional masks for the amygdala were created using the voxels (five or more contiguous voxels) that showed a significant group-by-time interaction from the repeated-measures analysis of variance (ANOVA). Masks for the orbitofrontal cortex and the subgenual anterior cingulate cortex were created using voxels from the baseline two-sample t tests. The percent signal change calculation allowed us not only to verify the results from the SPM5 analyses but also to account for other covariates in the analyses (baseline activation, gender, age, depression severity, and so on) using SAS, version 9.2 (SAS Institute, Inc., Cary, N.C.). Thus, separate 2×2 (group-by-time) repeated-measures ANOVAs in SPM5 and a mixed linear model analysis of repeated measures with the Kenward-Roger correction applied to the unstructured covariance model in SAS were conducted to examine the differences in amygdala, orbitofrontal cortex, and subgenual anterior cingulate cortex activations between depressed adolescents and healthy comparison subjects for the fearful > neutral contrast over 8 weeks. Post hoc t tests in SPM5 and one-way ANOVAs or one-way analyses of covariance in SAS were conducted to evaluate the significant group-by-time interaction simple effects at baseline and at week 8, respectively. Additionally, mean changes in activation and the effect size of the change (Cohen's d) from baseline to week 8 for each region were examined using mean contrasts. Similar mixed-model analyses and post hoc tests of simple effects were also carried out with age, gender, and handedness included as covariates. To compare our results with those of previous studies, we also ran all analyses again, this time excluding patients with comorbid anxiety disorders from the depressed group (N=13 at baseline and N=9 at week 8).
Finally, baseline demographic and clinical differences between depressed and healthy adolescents were compared using two-independent-sample t tests, with the Satterthwaite method for unequal variances (for continuous variables) and Fisher's exact test (for categorical variables). The significance threshold for all tests was set at a p value of 0.05 (two-tailed); to address multiple testing on the post hoc tests of simple effects, p values were adjusted using the false discovery rate (
25).
Results
The demographic characteristics of the depressed and comparison groups were similar (
Table 1). There were no significant differences between the groups in mean age or in gender distribution. In the depression group, the mean total score on the Children's Depression Rating Scale–Revised at baseline was moderately severe (mean=51.9, SD=7.6). By week 8, 60% (9/15) of the depressed adolescents had responded to treatment. The mean score on the Children's Depression Rating Scale–Revised at week 8 was 30.5 (SD=6.9) for the depression group and 18.0 (SD=1.2) for the healthy comparison group (p<0.001).
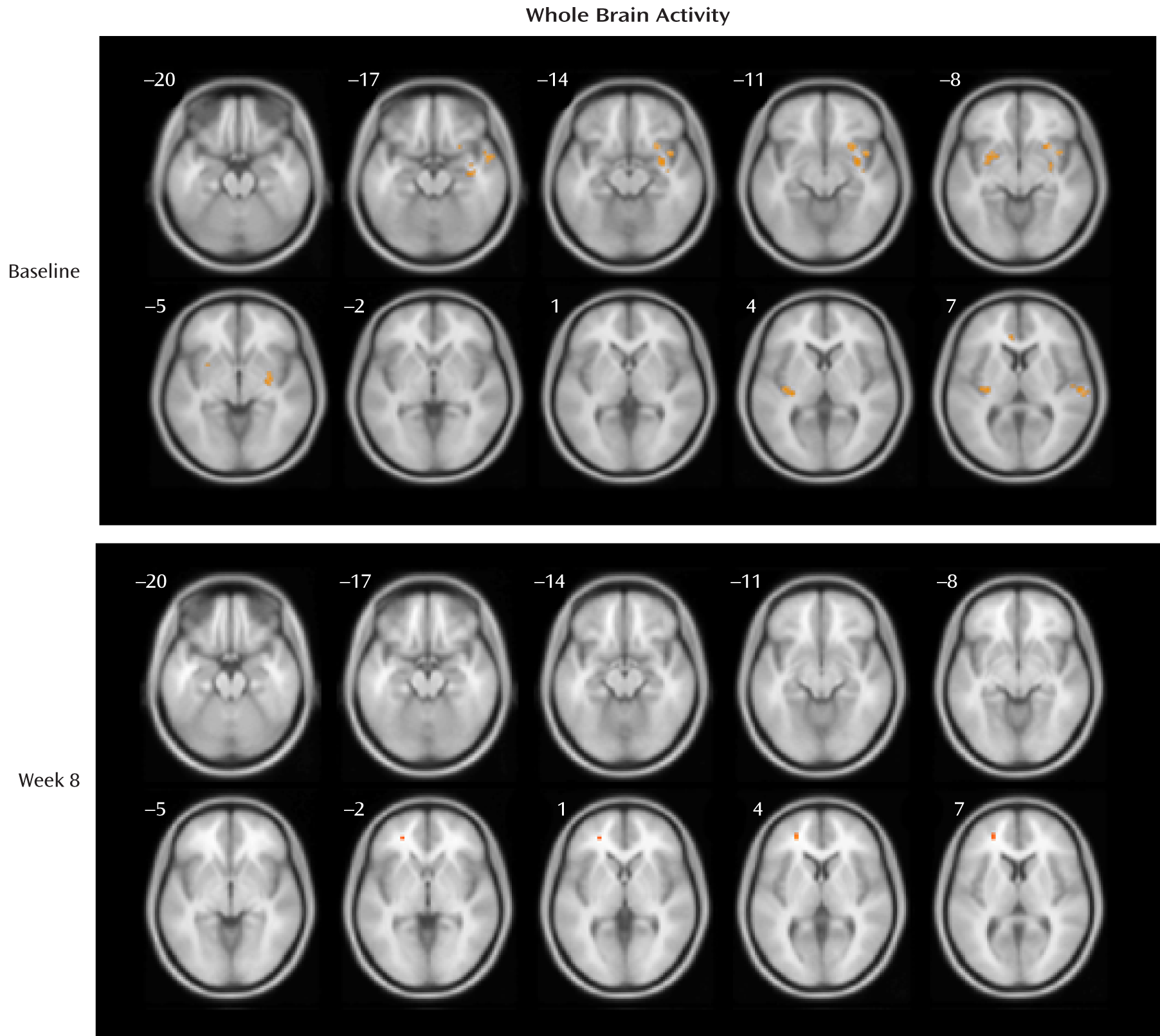
Whole Brain Analyses
At baseline, the depression group showed significantly greater activations relative to the healthy comparison group for the fearful > neutral contrast in the regions of the left and right frontal lobe, temporal lobe, putamen, insula, and cingulate gyrus and in the right amygdala, right hippocampus, and right occipital cortex (p values, <0.001), although none of the differences reached a p value of 0.05 after false discovery rate correction for multiple testing. At week 8, the depression group had greater activation than the comparison group (in a single five-voxel cluster) only at the left superior and middle frontal gyrus (MNI coordinates: x=–25, y=44, z=4; z=3.66, t=4.16, uncorrected p<0.001; false discovery rate corrected p=0.76). See
Table 2 for cluster size and MNI coordinates and
Figure 2 for activation maps.
Region-of-Interest Analyses
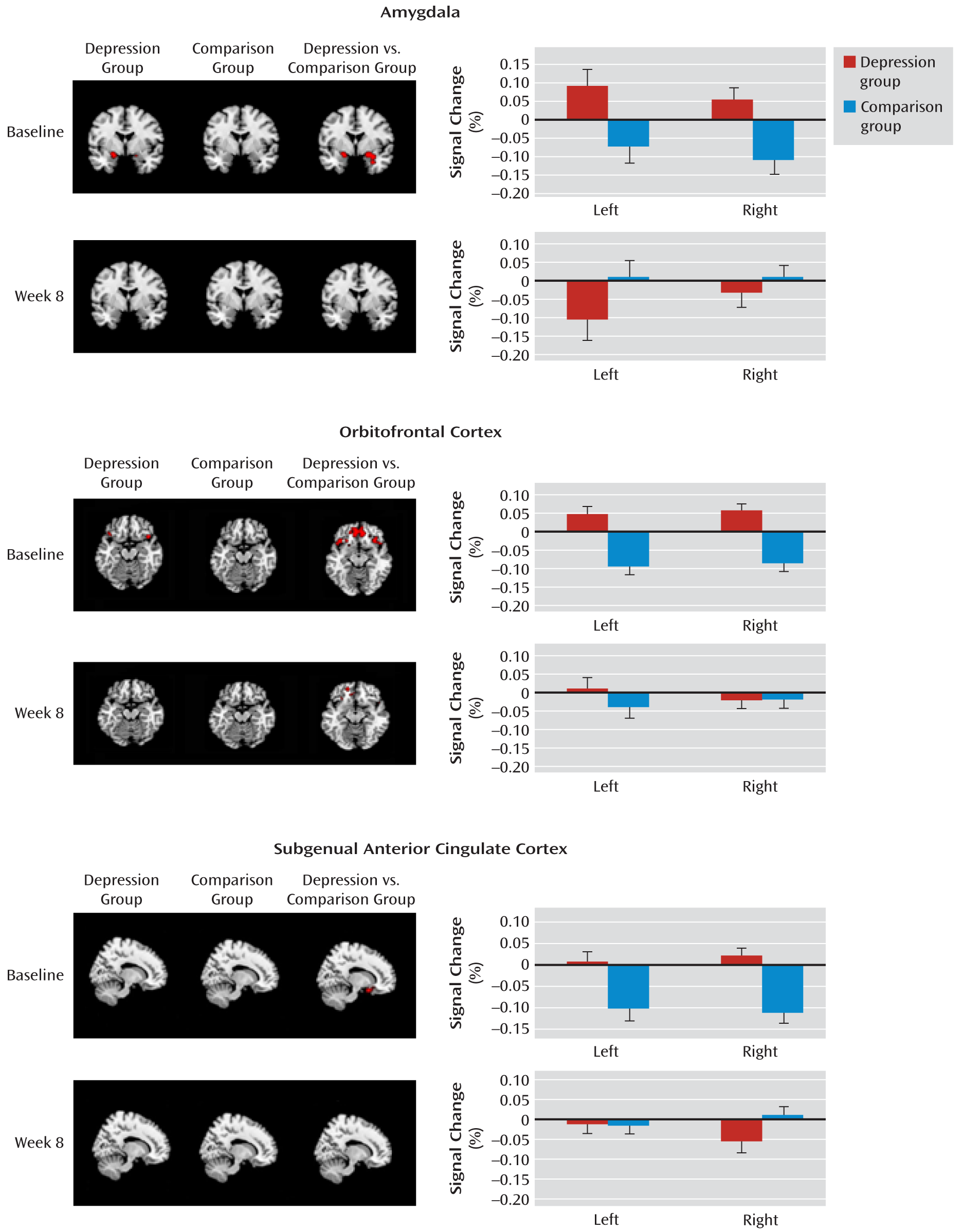
Amygdala.
The linear mixed-model analysis of repeated measures revealed a significant group-by-time interaction effect for left (F=8.56, df=1, 36.8, p=0.006) and right (F=7.69, df=1, 36.2, p=0.009) amygdala activations of the fearful > neutral contrast.
Figure 3 presents activation maps for the depression and healthy comparison groups and for the depression group relative to the comparison group for the fearful > neutral contrast, as well as the mean percent signal changes (least squares means) from the mixed-model analyses. At baseline, the depression group responded similarly to fearful and neutral faces, whereas the comparison group tended to show greater activation to neutral than to fearful faces in the right amygdala, although this difference fell short of statistical significance (p=0.07). At week 8, the depression and comparison groups responded similarly to fearful and neutral faces. The post hoc simple group effects indicated that (similar to adults) the depressed adolescents had greater activation than the healthy comparison subjects in both the left (F=5.76, df=1, 38, p=0.04; d=0.76) and right (F=9.31, df=1, 38, p=0.008; d=0.96) amygdala at baseline, but not at week 8, after adjusting for multiple testing and baseline activation. The depressed adolescents had decreased activation at week 8 relative to baseline; however, only the decrease in the left amygdala reached significance (left: F=8.56, df=1, 37, p=0.01; d=0.76; right: p=0.09). Interestingly, while left amygdala activation was stable from baseline to week 8 in the healthy comparison group (p=0.25), activation of the right amygdala was increased, although this increase fell short of significance (p=0.06). Table S1 in the data supplement that accompanies the online edition of this article presents cluster size and MNI coordinates, and Table S2 presents details of the analyses conducted with SAS.
Orbitofrontal cortex.
The linear mixed-model analysis of repeated measures revealed a significant group-by-time interaction effect for the right orbitofrontal cortex (F=10.43, df=1, 36.5, p=0.0026), while only a main effect for group was observed for the left orbitofrontal cortex (F=16.54, df=1, 37.9, p=0.0002). At baseline, the depression group showed a nonsignificantly (p=0.08) greater activation to fearful than neutral faces in the right orbitofrontal cortex, while the comparison group had a greater activation to neutral than to fearful faces in the orbitofrontal cortex bilaterally (p=0.01). At week 8, the depression and comparison groups responded similarly to fearful and neutral faces. The post hoc simple group effects confirmed that (similar to adults) depressed adolescents had greater activations than healthy comparison subjects in both the left (F=17.86, df=1, 38, p=0.0002; d=1.33) and the right (F=19.04, df=1, 38, p=0.0002; d=1.38) orbitofrontal cortex at baseline, but not at week 8. The decrease in activations from baseline to week 8 for depressed adolescents approached significance for the right (F=5.17, df=1, 36.7, p=0.06; d=0.78) but not the left orbitofrontal cortex. Healthy adolescents, on the other hand, had increased activation from baseline to week 8 in the right (p=0.04) but not the left (p=0.13) orbitofrontal cortex (
Figure 3; see also Tables S1 and S2 in the online data supplement).
Subgenual anterior cingulate cortex.
The linear mixed-model analysis of repeated measures revealed a significant group-by-time interaction effect for the left (F=5.04, df=1, 36.2, p=0.031) and right (F=14.99, df=1, 36.9, p=0.0004) subgenual anterior cingulate cortex. At baseline, depressed adolescents responded similarly to fearful and neutral faces, whereas healthy adolescents had greater activation to neutral than to fearful faces in the left and right subgenual anterior cingulate cortex (p=0.02). At week 8, depressed and healthy adolescents responded similarly to fearful and neutral faces. The post hoc simple group effects confirmed that depressed adolescents had greater activation than healthy comparison subjects in the left (F=10.10, df=1, 38, p=0.006; d=1.01) and right (F=13.73, df=1, 38, p=0.001; d=1.17) subgenual anterior cingulate cortex at baseline, but not at week 8. The decrease in subgenual anterior cingulate cortex activations from baseline to week 8 for depressed adolescents approached significance for the right (F=4.35, df=1, 37.1, p=0.08) but not the left side. Healthy adolescents had increased activation from baseline to week 8 in the subgenual anterior cingulate cortex bilaterally (left: p=0.007; right: p=0.002; see
Figure 3 and Tables S1 and S2).
The above analyses were also run to include as covariates age, gender, and handedness in the model, with similar results (data not shown). We again obtained similar results when we excluded depressed adolescents who had a comorbid anxiety disorder (data not shown).
Discussion
We report that depression in adolescents involves similar brain regions to those affected in adult depression. Like adults with depression (
3,
4), untreated depressed adolescents demonstrated greater activations to fearful than to neutral faces in limbic regions (the amygdala, orbitofrontal cortex, and subgenual anterior cingulate cortex) compared with healthy adolescents. However, these data do not show a hypofrontal activation pattern, as reported in some adult depression studies (
6,
7), but rather show increased frontal activity. This could reflect a compensatory mechanism responding to increased limbic inputs because of the emotional nature of the task we used. Frontal hypoactivity may be seen only when cognitive processes (i.e., attention and memory) are in demand.
To our knowledge, this is the first study to report changes in brain activity in adolescents after antidepressant treatment. After 8 weeks of fluoxetine treatment, brain activation to emotional faces in depressed adolescents normalized to activation levels seen in healthy adolescents. This evidence of normalization of brain function is an important finding and should, to some extent, mitigate the safety concerns about the risk of antidepressant use in the pediatric population.
This is also the first study, to our knowledge, to use repeat fMRI assessment of healthy comparison subjects, as well as repeat assessment of the depressed adolescents, thus providing assessment of expected test-retest reliability. This strategy provides a more reliable approach to identifying differential brain responses to fearful versus neutral facial expressions between depressed and healthy adolescents. Interestingly, healthy adolescents showed a greater brain activation to neutral faces than to fearful faces when they encountered the stimuli for the first time (an observation that reached significance for the orbitofrontal cortex and the subgenual anterior cingulate cortex even after false discovery rate correction for multiple testing). Only after a repeated exposure to the faces, when the stimuli were no longer novel, did healthy adolescents have similar responses to fearful and neutral faces. One explanation for this interesting finding is that the neutral faces are more ambiguous or more interesting to adolescents than the fearful faces, thus generating a greater response. This could be the reason that the differences between activations to fearful and neutral faces were relatively small for depressed adolescents. Future studies that use scrambled faces as contrasts to emotional faces may reveal greater activation differences between emotional and neutral stimuli.
Although this study provides unique data for considering depression in adolescents, it has several limitations. First, we only used two types of emotional faces and used neutral faces as a contrast to fearful faces. We did not evaluate positive emotions. It may be interesting to explore the use of faces showing positive emotions in future research, since depressed adults have biased perceptions toward positive emotional expressions, tending to perceive happy emotions as sad (
26). In addition, relative to healthy comparison subjects, depressed adults show an attenuated limbic response to happy faces, an alteration that is reversed after antidepressant treatment (
27). Future studies of positive emotions can provide information about the processing of positive emotional information in depressed compared with healthy youths and about how these processes may contribute to depressive symptoms and response to treatment.
Second, our depression group included patients with comorbid psychiatric disorders, such as anxiety disorders. While this naturalistic approach reflects the composition of the target population, one could argue that the imaging outcomes may be moderated by the comorbid symptoms rather than solely by depression. However, including comorbid psychiatric disorders as a covariate in the analyses had no effect, which suggests that this is not the case. The inclusion of patients with comorbid conditions does make the findings more generalizable, as between 50% and 90% of depressed youths have a comorbid disorder (
1). Third, the effect of improvement in depression is confounded by the medication effect, a confound that is very difficult to disassemble. Future studies using multiple fMRI assessments at different stages of treatment may help us sort out this issue.
Despite these limitations, our study provides strong evidence that treating depression leads to normalization of brain activity and response to negative emotions in depressed adolescents. Our findings establish depression in youths as substantially similar to depression in adults on a neurobiological basis. They also show that the effects of antidepressant medication on brain activation are similar in adolescents and adults. Furthermore, brain activations may serve as a biomarker for response to treatment for depression in youths, a possibility that needs to be explored in future studies.
Acknowledgments
The authors thank Jarrette Moore, M.A., and Uma Yezhuvath, Ph.D., for their important involvement in the study.