Converging evidence from postmortem, magnetic resonance imaging (MRI), and head circumference studies has documented that individuals with autism spectrum disorders (ASDs) have larger brains and heads than comparison subjects (
1–
10). Brain MRI studies have revealed generalized enlargement in cerebral cortical gray and white matter (
9–
11) and selected subcortical structures (
12) that is present as early as 2 years of age. A longitudinal MRI study of 2-year-old children with ASDs followed up at 4–5 years of age demonstrated enlargement at age 2 with no increase in growth rate during the subsequent 2-year interval (
10), and this pattern is consistent with the conclusion that high brain volume is the result of overgrowth occurring before 2 years of age. A recent study of a group of 2–50-year-olds with ASDs (
13) also showed that early brain enlargement was associated with autism and that it was characterized by an early postnatal period of overgrowth followed by a period of slower or arrested growth.
Longitudinal studies of head circumference suggest that the onset of brain overgrowth is in the latter part of the first year of life (
9,
14), and high rates of macrocephaly have been observed in parents of probands with ASDs (
7). Data from behavioral studies of infant siblings of autistic individuals (infant siblings who are therefore at high genetic risk for developing ASDs) suggest that most of the defining features of this condition (e.g., social deficits) are not present at 6 months of age but are first evident by 12 months of age (
15). These two independent lines of research (brain studies, developmental behavioral studies) appear to converge on the latter part of the first year of life as a period of both changes in brain structure and the onset of behavioral symptoms of autism.
Structural imaging studies of early brain development in typically developing children highlight that the first year of life is a time of dynamic growth, where total brain volume increases 100% (
16). As autism is not typically diagnosed until about age 2 years or later, studies of abnormal brain development during infancy in autism must wait until the diagnosis is established. Given the high recurrence risk of autism in subsequently born siblings (
17), a strategy that has recently been employed is the examination of the infant siblings of autistic children (i.e., infants at risk for autism before the diagnosis of autism). Virtually nothing is known about early brain development in infants at risk for autism from neuroimaging studies. It could be valuable to know whether there are observable differences in brain volume between these high-risk infants and infants from the normal population who have no known genetic risk for autism and therefore most often display typical development, i.e., “low-risk” subjects.
In the present study we assessed volumetric brain MRI measures in a large group of 6-month-old infants at high genetic risk for autism and in infants at low risk for autism. To our knowledge, this study is the first to measure brain volume in 6-month-olds at high familial risk for autism, approximately 20% of whom may receive a later diagnosis of an ASD (
17), to test whether brain volume differences may be associated with high risk for ASDs.
Method
Study Group
This investigation included data acquired from an Autism Center of Excellence network study funded by the National Institutes of Health. Informally called the Infant Brain Imaging Study (IBIS), the network includes four clinical data collection sites (University of North Carolina at Chapel Hill, University of Washington, Children's Hospital of Philadelphia, Washington University in St Louis), a data coordinating center (Montreal Neurological Institute at McGill University), and two image processing sites (University of Utah, University of North Carolina). The network study collects neuroimaging and behavioral data on infants who are at genetic risk for ASDs by virtue of having an older sibling with autism, referred to as “high-risk infant siblings.” The study enrolls 1) 6-month-old high-risk infant siblings who are seen for follow-up assessments at 12 and 24 months of age, 2) high-risk infant siblings who enter the study at 12 months of age and are followed up at 24 months, and 3) comparison subjects who are typically developing infants considered to be at low risk for autism (i.e., an older sibling is developing typically), who are seen at 6, 12 and 24 months. Diagnostic outcome data for ASD status is determined at 24 months of age (and again at 36 months of age if the timeline of the project allows). For the purposes of this article, we have included cross-sectional data on the 6-month-olds who were scanned from the start of the study through the fall of 2010. Two groups were included: infants at high risk for autism and typically developing children considered to be at low risk for autism. Subjects were combined from all four clinical data collections sites.
The subjects include all children with imaging data collected and processed through the end of September 2010. There were 318 children who attempted the MRI scans, and 88% of the high-risk infants and 83% of the low-risk subjects were successfully scanned, for a total of 276 scans. Owing to difficulties in scheduling visits and missed visits (e.g., due to illness, changes in travel plans), some children were seen in a wider age window, up to 8 months of age. Infants develop rapidly during the first year, and so for the purpose of this analysis, a narrow, optimal age window was defined as from 1 week before age 6 months to 3 weeks after age 6 months. There were 134 infants who were in this optimal age window (98 high-risk, 36 low-risk), and all results are based on this group. Of note, the wider age group at the first time point will be included in later longitudinal analyses of these data.
Subjects were characterized as having high risk if they had an older sibling with a diagnosis of an ASD that was documented in a clinical diagnostic report and confirmed by the Autism Diagnostic Interview-Revised (18) administered at enrollment. Subjects were enrolled in the low-risk group if they had an older sibling without evidence of ASDs and no family history of a first- or second-degree relative with an ASD. Exclusion criteria for both groups included the following: 1) diagnosis or physical signs strongly suggestive of a genetic condition or syndrome reported to be associated with ASDs (e.g., fragile X syndrome), 2) significant medical or neurological condition affecting growth, development, or cognition (e.g., CNS infection, seizure disorder, congenital heart disease), 3) sensory impairment, such as vision or hearing loss, 4) low birth weight (<2,000 g) or prematurity (<36 weeks gestation), 5) possible perinatal brain injury from exposure to in utero exogenous compounds reported to likely affect brain adversely in at least some individuals (e.g., alcohol, selected prescription medications), 6) non-English-speaking family, 7) contraindication for MRI (e.g., metal implants), 8) adoption, and 9) family history of intellectual disability, psychosis, schizophrenia, or bipolar disorder in a first-degree relative.
Assessment Protocols
Behavioral assessment.
The infants were assessed directly at ages 6, 12, and 24 months, and a telephone interview was conducted with the parents at 18 months to assess their infant's development. The direct assessments included brain MRI scans in addition to a battery of behavioral and developmental tests. The assessment battery for the visit at 6 months included the Mullen Scales of Early Learning (
19), the Vineland Adaptive Behavior Scales-II (
20), the Autism Observation Scale for Infants (
21), various questionnaires examining behavior, temperament, and family characteristics, and a medical record review. See
Table 1 for a summary of subject characteristics.
MRI acquisition.
All the brain MRI scans were completed at each of the clinical sites on a 3-T Siemens Tim Trio scanner with a 12-channel head coil. All scans were completed while the infants were naturally sleeping. A specific structured preparation was completed by the families at home before the scanning; it included conditioning to the scanner sounds on a compact disc played to the infants while sleeping. The imaging protocol was designed to maximize tissue contrast for volumetric analysis across three time points (ages 6, 12, 24 months). The protocol included 1) a localizer scan, 2) a three-dimensional T1 magnetization-prepared rapid acquisition gradient-echo scan: TR=2,400 ms, TE=3.16 ms, 160 sagittal slices, field of view (FOV)=256 mm, voxel size=1 mm3, 3) a three-dimensional T2 fast spin echo scan: TR=3,200 ms, TE=499 ms, 160 sagittal slices, FOV=256 mm, voxel size=1 mm3, and 4) a 25-direction diffusion tensor imaging scan: TR=12,800 ms, TE=102 ms, slice thickness=2 mm isotropic, variable b value=maximum of 1,000 s/mm2, FOV=190 mm.
A number of quality control procedures were employed to assess scanner stability and reliability across sites, time, and procedures. A LEGO phantom (
22) was scanned monthly at each location and analyzed to assess image quality and quantitatively address site-specific regional distortions. Two adult subjects (“human phantoms”) were scanned once per year per scanner (twice in year 1). The data for these phantoms were evaluated for scanner stability across sites and time (
23). The results indicated excellent stability across sites, with covariates of variation for intracranial volume below 1% and with intraclass correlations for intracranial volume at 0.98 for intersite and 0.99 for intrasite reliability. Finally, all scans were blindly reviewed for image quality by a single rater (D. Louis Collins, McGill University) and again rated by a single reviewer (Rachel Gimpel Smith, University of North Carolina) in a preprocessing stage before image analysis.
Radiologic review.
All scans were reviewed locally by a pediatric neuroradiologist for radiologic findings that, if present, could be communicated to the participants. In addition, a board-certified pediatric neuroradiologist (R.C.M., Washington University) blindly reviewed all MRI scans across the IBIS network and rated the incidental findings. A third neuroradiologist (D.W.W.S., University of Washington) provided a second blind review for the Washington University site and contributed to a final consensus rating if there were discrepancies between the local site reviews and the network review. The final consensus review was used to evaluate whether there were group differences in the number and/or type of incidental findings.
Image processing.
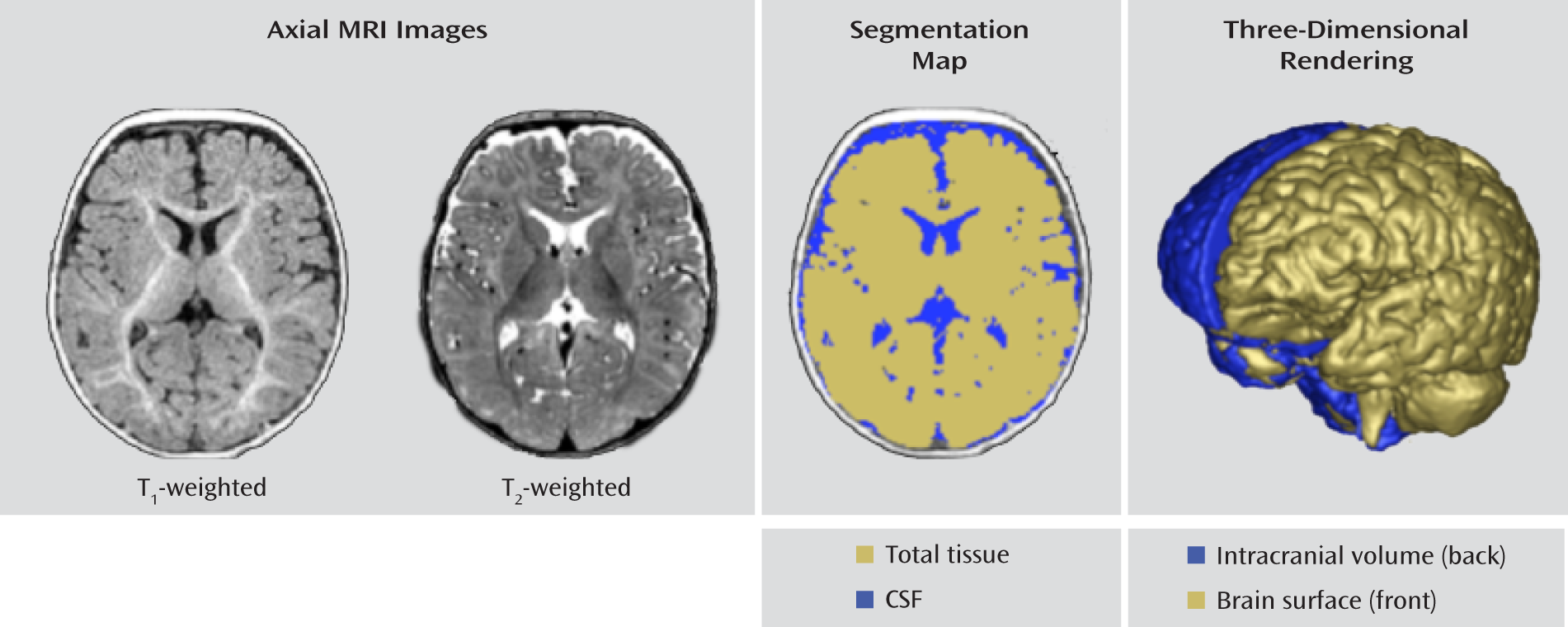
The following brain volumes were obtained: intracranial, total brain (gray matter plus white matter), total CSF, cerebrum, cerebellum, and lateral ventricles. There is minimal tissue contrast in the 6-month-old brain because of ongoing myelination and maturation. As a result, reliable separation of white matter and gray matter tissue is highly limited. Thus, we chose to initially focus on the total brain volume measure without separating white and gray matter. We also parcellated the brain into large regions (e.g., cerebrum, cerebellum, hemispheres) but omitted a fine-grained lobar or cortical segmentation, which is less reliable at this young age.
The brain volume segmentations were obtained following a rigid transformation to a normative brain space and preprocessing for bias correction and intensity normalization. These steps are part of the expectation-maximization (EM) tissue segmentation method (
24) using the AutoSeg tool (
25). Tissue probability maps for white matter, gray matter, and CSF were obtained. Intracranial volume was defined to be the sum of white matter, gray matter, and CSF, and an example of the segmentation labeling is shown in
Figure 1.
Measures of head circumference were obtained by using a semiautomated image processing tool according to an established protocol (
9). Head circumference was measured by a single rater using the HeadCirc tool developed by collaborators at the University of North Carolina. HeadCirc computes head circumference by extracting the appropriate contour from head segmentation of MRI images (using Fourier harmonics to parameterize the contour of the head on MRI). Intra- and interrater reliability for the head circumference measures in the data set from the 6-month-old infants was exceptional, with an intraclass correlation (ICC) of 0.99 for each one. The ITK-SNAP tool (
26) was used by a single rater to obtain measurements of the lateral ventricles. ICC values for intra- and interrater reliability for the lateral ventricles were 0.99 (for combined volume and for right and left hemispheres). The AutoSeg, HeadCirc, and ITK-SNAP tools are available at
http://www.ia.unc.edu/dev/download and
http://www.nitrc.org.
Scans from 12 infants were excluded because they did not pass the initial scan review and/or preprocessing steps. The problems included misalignment (N=2), off-center or distorted image (N=2), poor registration (N=3), and poor image quality or artifact (N=5). There were no group differences in the rate or type of scan failures.
Statistical Analysis
Power consideration.
The design of the study was based on findings from our previous comparison of 2-4-year-olds with ASDs and typically developing comparison subjects (
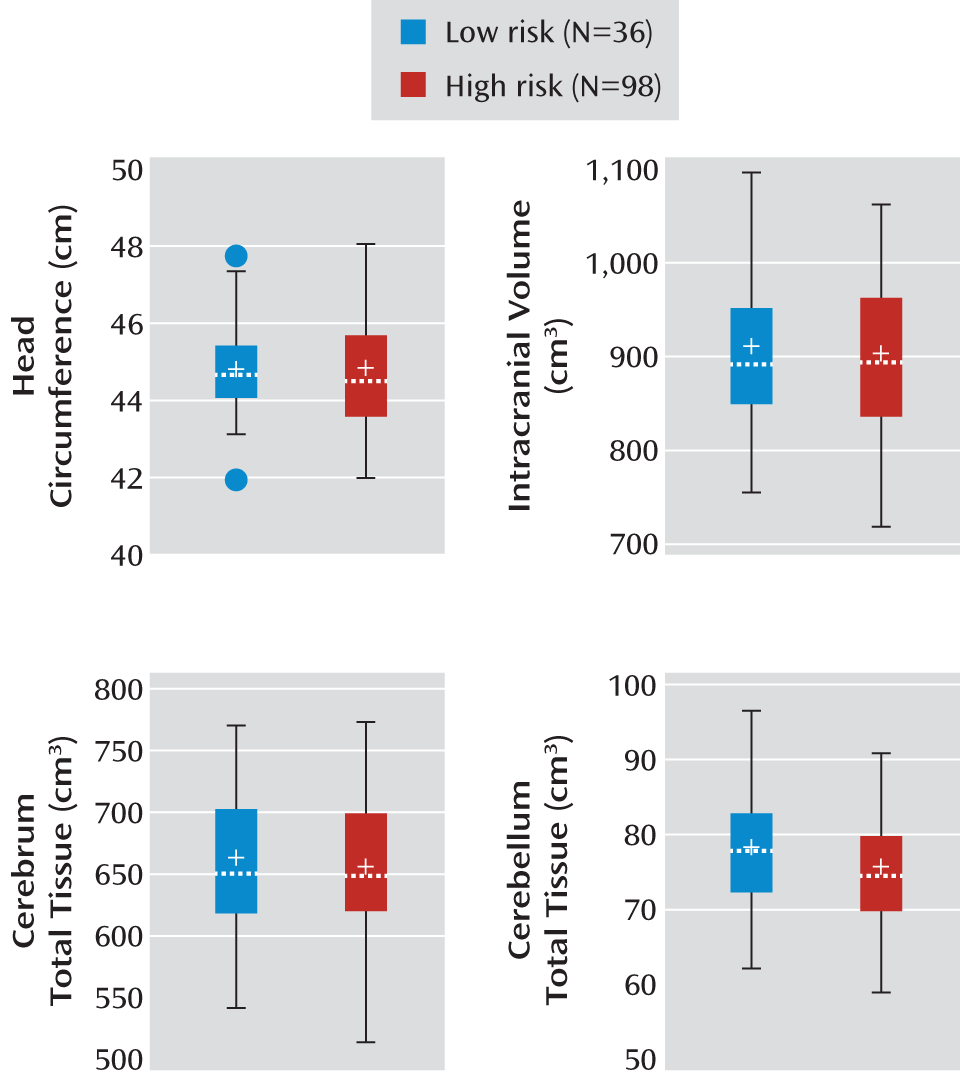
10). The percentage difference in the volume of relevant structures (e.g., cerebral cortex, cerebral cortical white matter, and temporal lobe white matter) was 4% to 9%, with effect sizes (Cohen's d) from 0.59 to 0.95, in cross-sectional groups of 45–51 children with ASDs and 15–26 comparison subjects. With our current groups of 36 low-risk and 98 high-risk infants, we expected to have 85%–99% power to achieve effect sizes similar to those in the 2–4-year-old children with ASDs.
Analysis.
Our research goal was to examine whether there were significant group differences in head circumference, brain volumes, and radiologic findings in a group of infants at high genetic risk for autism. Group differences in sex were tested with Fisher's exact test, and separate analysis of variance (ANOVA) models were used to assess group differences in the Mullen early learning composite score, gestational age at birth, age at MRI scan, and maternal age at birth while controlling for site differences. Cross-sectional group differences in brain volumes were analyzed by using a general linear model in SAS (SAS Institute, Cary, N.C.). Covariates included in the model were age at scan, sex (male/female), site, and the Mullen early learning composite standard score. The dependent variables of interest included head circumference, intracranial volume, and volumes of the total tissue (gray plus white matter), CSF, cerebrum (left and right total tissue plus CSF), cerebellum (left and right total tissue plus CSF), and left and right lateral ventricles. To test the influence of sex differences on group effect, we included the additional interaction term (group by sex) in the model.
Discussion
First-degree relatives of autistic individuals are reported to have high rates of macrocephaly (
6). In addition, it is anticipated that approximately 20% of high-risk infants in this group will meet criteria for an ASD by 36 months of age (
17). To our knowledge, this is the first report of brain volume in 6-month-old infants at high familial risk for autism from an ongoing longitudinal neuroimaging study. We found no differences between these infants and low-risk peers in overall brain tissue volumes, head circumference, or ventricular volumes.
This investigation does not address the question of whether early brain volume differences can be observed in high-risk children who are later diagnosed with an ASD, which will be examined in the longitudinal data set from this study. Rather, our intent was to study whether differences in brain volume could be detected in children at high genetic risk for autism, an approach that has been used previously to examine familial rates of macrocephaly in ASDs (
7). There is, therefore, a chance that we will be able to detect significant differences at 6 months once we are able to examine trajectories of growth in the various subgroups at 24 months of age.
The absence of brain volume differences in 6-month-olds at high familial risk for autism in this large cohort is consistent with our hypothesis that brain enlargement in autism and possibly in those with high genetic liability is a later-occurring postnatal process. Disruptions of cortical maturation related to impaired experience-dependent synaptic development have been observed in mouse models of both Angelman syndrome (
27) and fragile X syndrome (
28), neurogenetic developmental disorders with behavioral features that overlap with those of ASDs. There has also been a report of mutations associated with the defective expression of activity-driven genes in individuals with ASDs (
29), although it is unclear whether this mechanism accounts for more than a very small proportion of genetic variance underlying ASDs. Other hypotheses about brain overgrowth, based on the observation that it is associated with increases in cortical surface area (
10), suggest that brain overgrowth in the latter part of the first year may be the result of an increase in neuronal precursors, perhaps related to aberrant molecular regulatory processes (
30). The findings from the current study suggest that early postnatal events may underlie later-occurring brain overgrowth and raise the possibility that there is a window of opportunity in which early postnatal intervention, during a period of tremendous brain plasticity, may have an important impact on later emergence of autistic behavior.
Studies examining early behavioral markers of autism have shown that infants who later go on to receive a diagnosis of an ASD do not exhibit overt autistic behaviors at 6 months of age (
15,
17). However, by 12 months of age, a time when we anticipate brain differences may begin to emerge, behavioral deficits can be detected in the infants who are later diagnosed with autism (
15,
17,
31). Our group is currently examining the behavioral characteristics of this study group. In the current study, the high-risk group likely includes children who will later receive a diagnosis of autism. With the later addition of diagnostic outcome data for this group, it will be possible to examine whether brain volumes for children in the high-risk group who eventually manifest ASDs differ from volumes for high-risk children who do not go on to develop ASDs, to specifically test our hypothesis of brain overgrowth in the latter part of the first year of life or early in the second year of life.
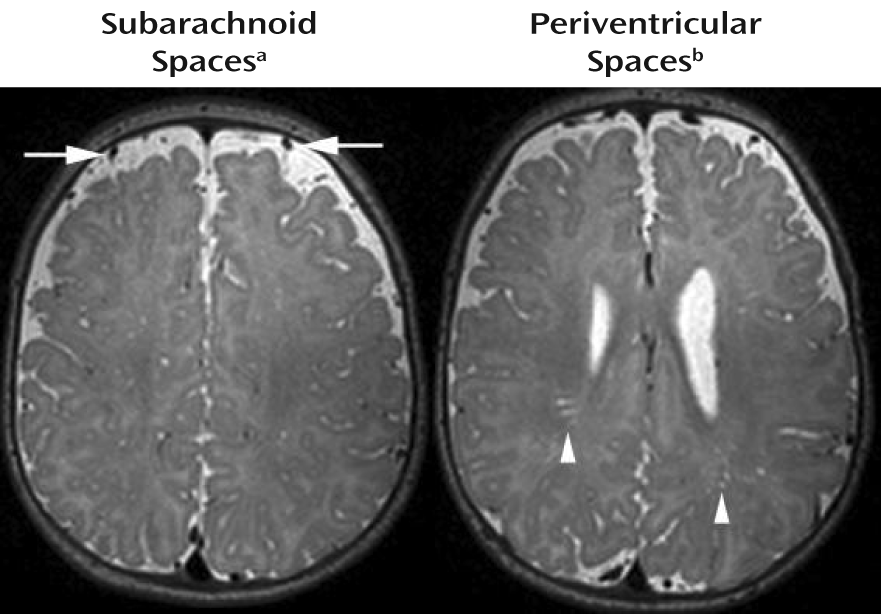
We also did not detect any clinically significant radiologic findings specific to the high-risk group. The types and rates of incidental findings observed were similar in the two groups, as were the degrees of enlargement for the subarachnoid spaces and the periventricular spaces. Most investigations of clinical neuroradiologic abnormalities in individuals with autism have not detected a significant type or pattern of findings associated with the disorder. Scattered reports of individuals with neuronal migration abnormalities (
32), subcortical abnormalities in the corpus callosum (
33), and high rates of dilated Virchow-Robin spaces (
34) exist. However, interpretation of this research has been complicated by inconsistent findings across studies and by numerous methodological shortcomings, such as the etiologic heterogeneity known to be inherent in autism, small study groups, and variations in subject age. One large-scale retrospective study (
35) showed higher rates of clinically reported abnormalities in children with autism than in patients without developmental or neurological disorders. Forty-eight percent of the children with ASDs were rated as having abnormalities. The primary abnormalities included white matter signal intensity abnormalities, dilated Virchow-Robin spaces, and temporal lobe abnormalities (e.g., subcortical hyperintensities in the temporal poles). White matter abnormalities (e.g., punctate or posterior T
2 hyperintensities) were present in younger children (e.g., 5-year-olds versus 7-year-olds), while the temporal lobe abnormalities showed no developmental trend. It should be noted that the current study differs from the investigation by Boddaert et al. (
35); whereas they studied children 2 to 16 years of age with ASDs, we examined infants at high risk for autism. The developmental nature of the incidental findings we observed and their association with ASDs therefore remain questions worthy of future investigation.
A limitation of this study, due in part to our focus on 6-month-olds, is that because of the difficulty associated with segmenting gray and white matter at this early age in development, we were unable to define regionally specific brain volumes. White matter structure and myelination may best be captured with other methods, such as diffusion tensor imaging, and we recently reported significant differences in white matter development between high-risk infants who did and did not later receive a diagnosis of an ASD (
36). More dynamic methods, such as resting-state functional MRI, may also detect early functional changes before the occurrence of more grossly observable changes in volume. These methods may be more sensitive to early brain differences and may be able to detect abnormalities in structural or functional development that are not possible to identify by means of standard tissue classification methods. Last, our current study was cross-sectional, and at this point the study group is characterized in terms of high-risk and low-risk status. Only a portion of our current high-risk group may later be diagnosed with an ASD. We will include diagnostic outcome data and longitudinal imaging data from later time points (e.g., 12, 24, and/or 36 months of age) to examine the groups for more specific information on the behavioral and biological mechanisms underlying the development of ASDs.
Acknowledgments
The Infant Brain Imaging Study (IBIS) Network is an NIH-funded Autism Center of Excellence and consists of a consortium of seven universities in the United States and Canada. Clinical sites are located at the University of North Carolina (J. Piven, IBIS Network primary investigator; H.C. Hazlett, C. Chappell); the University of Washington (S.R. Dager, A.M. Estes, D. Shaw); Washington University (K.N. Botteron, R.C. McKinstry, J. Constantino, J. Pruett); Children's Hospital of Philadelphia (R.T. Schultz, S.J. Paterson); and the University of Alberta (L. Zwaigenbaum). The data coordinating center is at the Montreal Neurological Institute (A.C. Evans, D.L. Collins, G.B. Pike, V. Fonov, P. Kostopoulos, S. Das). The image processing core is at the University of Utah (G. Gerig) and the University of North Carolina (M. Styner). The statistical analysis core is at the University of North Carolina (H. Gu). The genetics analysis core is at the University of North Carolina (P. Sullivan, F. Wright).
The authors thank Sun Hyung Kim, Rachel Gimpel Smith, Michael Graves, and Ryan Scotton for assistance in processing these data; Penelope Kostopoulus and Samir Das for assistance with database management; and the families who participated in this study.