The incidence and period prevalence of major depressive disorder during pregnancy are 7.5% and 12.7%, respectively (
1). Pregnant women often discontinue both psychotherapy and pharmacotherapy and do not resume care after birth (
2,
3). Pregnant women who stop their medication proximate to conception have a higher risk for relapse (68%) than do those who maintain treatment (26%) (
4). These low treatment and high relapse rates are juxtaposed against mounting evidence that major depression increases the risk for adverse pregnancy outcomes.
During pregnancy, depression is associated with risk directly related to the physiological dysregulation of psychiatric disorder as well as to associated maternal behaviors, such as smoking, poor nutrition, substance abuse, inadequate obstetrical care, and interpersonal isolation and suicide (
5). Women with antenatal depression have higher rates of giving birth to infants who are small for gestational age (
6) and preterm (
7). In a meta-analysis, Grote et al. (
8) found that major depressive disorder or clinically significant depressive symptoms were associated with a 39% increase in the relative risk for preterm birth, a 49% increase for low birth weight, and a 45% increase for intrauterine growth restriction. Several causal pathways have been suggested, such as dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis, increased uterine artery resistance with placental hypoperfusion in response to stress (
9), and the exaggerated inflammatory responses associated with depression (
10).
In contrast, studies of the influence of postpartum depressive symptoms on young children’s growth have not shown a significant relationship (
6,
11,
12). Ramsay et al. (
13) performed a prospective study of the impact of maternal depressive symptoms on neonatal sucking and found no effect on feeding practices, infant feeding abilities, or growth.
Antidepressant treatment of pregnant women has also been associated with adverse outcomes. Meta-analyses have shown that women treated with selective serotonin reuptake inhibitors (SSRIs) have a two- to threefold greater risk for preterm birth and a higher rate of delivering low-birth-weight infants than do women not exposed to SSRIs (
14,
15). In our previously published prospective investigation (
7), preterm birth rates were similar in women exposed continuously to depression (without SSRI treatment) and those exposed to SSRIs (23% and 21%, respectively). These rates were significantly higher than the rate for women with neither exposure (6%). In that study (
7), measurements at birth of weight, length, and head circumference (corrected for gestational age) did not significantly differ (
7). In a recent study by El Marroun et al. (
16), maternal depression was associated with slower rates of fetal body and head growth, while in pregnant women treated with SSRIs there was no impact on fetal body growth, but there was delayed head growth and a higher rate of preterm birth.
Few data exist on the later physical growth of infants following in utero exposure to depression or SSRIs, and the available studies did not include a comparison group of depressed women without antidepressant exposure. In women treated with SSRIs or serotonin-norepinephrine reuptake inhibitors, relative to untreated comparison subjects (
17), antenatal drug exposure was associated with lower infant birth weight, shorter birth length, and smaller head circumference, and these differences persisted at 1 month of age (
18). Infants born to women continuously treated with SSRIs (N=21) or comparison subjects (N=20) were evaluated on the HPA and insulin-like growth factor 1 (IGF-1) axes for relationships of these variables to growth and hormonal profiles. The SSRI-exposed infants had significantly shorter length and smaller head circumference. SSRI-exposed infants also had a significantly lower cord blood level of cortisol and a higher level of thyroid-stimulating hormone. Placental IGF-1 receptor expression was significantly higher in the SSRI group than in the comparison group, and the urine level of 5-hydroxyindoleacetic acid (the major metabolite of serotonin) was negatively correlated with birth weight and with dehydroepiandrosterone level. The authors concluded that fetal exposure to SSRIs resulted in impaired intrauterine growth accompanied by alterations in the IGF-1 and HPA axes. However, no evidence that prenatal SSRI exposure reduced neonatal bone quality was observed (
19), although head circumference (but not birth weight or length) was significantly smaller in the SSRI-exposed infants than in the comparison group in the same investigation.
Another factor for consideration is postbirth exposure to SSRIs through breast milk, which could affect infant growth. However, the method of infant feeding (i.e., breast milk compared with formula) also has a substantial impact on infant growth. Compared with formula-fed infants, breast-fed infants have faster growth in the first few months of life, followed by slower growth in the last half of the first year. Breast-fed infants weigh less than formula-fed infants at 12 months (
20,
21).
In a retrospective cohort study, Chambers et al. (
22) found that infants of women who took the SSRI fluoxetine during pregnancy and lactation had less optimal growth than women who took fluoxetine during pregnancy
but not during breast-feeding. Infants who received fluoxetine through breast milk had poorer weight gain in the first 6 months of life than breast-fed infants of unmedicated mothers. However, the amount of fluoxetine exposure to women during pregnancy was also greater in those who continued taking the drug during lactation. Ten percent of the women in the group that was unmedicated during breast-feeding were treated with antidepressants in the third trimester, compared with 100% in the group who took fluoxetine during breast-feeding.
Consideration of the impact of both antenatal SSRI and depression exposures on fetal and infant growth is an understudied component of the risk-benefit decision process for developing treatment plans for depressed pregnant women (
23). Epidemiologic studies have demonstrated that infants born either small or large for gestational age have higher rates of chronic illnesses such as diabetes and cardiovascular disease as adults (
24,
25). Differentiating between the contribution of major depression or depressive symptoms and that of antidepressant treatment to growth presents a clinical conundrum (
8) because 1) investigators usually study the effects of one exposure without controlling for the other, 2) antidepressant use occurs at different antenatal times and dosages, 3) recognition and treatment of depression by the physician is associated with symptom severity and episode duration, and 4) depression is associated with use of other prescription and over-the-counter drugs as well as multiple confounders, such as smoking, substance use, overweight/obesity, and elevated life stress. Evidence from meta-analyses (
8) and other sources (
7) suggests that untreated antenatal depression is as likely to be associated with poor birth outcomes as is treatment with SSRIs. To our knowledge, no study of the impact of SSRI on infant growth has included a comparison group of unmedicated women with depression.
The aim of this study was to compare the measurements of weight, length, and head circumference among infants born to pregnant women treated with SSRIs, women with depression but no SSRI treatment, and women with neither exposure through 1 year postpartum. We hypothesized that no significant difference in anthropometric measurements would be observed among the infants of these three groups of women after control for key variables.
Method
The subjects in this analysis were derived from a parent study on antidepressant use during pregnancy. Details have been previously published (
7). In this prospective observational study, women were evaluated at weeks 20, 30, and 36 of gestation. The mother and infant pairs were assessed at 2, 12, 26, and 52 weeks postpartum.
Subjects
Pregnant women ages 15–44 years of age were enrolled at or before week 20. Women were eligible to participate if they had DSM-IV major depressive disorder with or without antidepressant treatment or if they did not have depression or antidepressant exposure. Consultation about depression management during pregnancy was provided to each depressed subject, and a summary letter was sent both to the woman and to her physician(s) as a benefit of participation. Enrollment did not depend on acceptance of consultation recommendations or choice of treatment during or after pregnancy in this observational study. Medications were managed by the women’s physicians, and no psychiatric treatment was prescribed by the study team. Pregnant women with psychosis, bipolar disorder, active substance use, or any antenatal exposure to benzodiazepines and women taking prescription drugs included by the Food and Drug Administration in class D or X were excluded. Women with multiple births or chronic medical illnesses, such as insulin-dependent diabetes, also were excluded. Subjects were recruited through physician referral, advertising, self-referral, and screening within the obstetrical ultrasound suite. Written informed consent was obtained from all subjects.
Descriptive data for the child-bearing study group included demographic variables (age, race, education, employment, marital status) and clinical characteristics (prepregnancy body mass index [BMI], parity, smoking status, alcohol intake, presence of a lifetime DSM-IV anxiety disorder, depression symptom score on the 29-item Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement [SIGH-ADS] [
26). The delivery and infant data included the rate of preterm birth (<37 weeks gestation [
27]), infant sex, growth measurements (weight, length, and head circumference) at birth, and breast-feeding status. Duration of gestation and newborn clinical status data were collected from the maternity and pediatric records by independent evaluators who were blind to the study hypotheses and design. The measure used was the Peripartum Events Scale (
28).
Exposures
Beginning with conception, antidepressant exposure was documented by charting the subjects’ drug doses across each week of gestation. The vast majority of the women were treated with SSRI antidepressant monotherapy; only two were treated with a second antidepressant, either bupropion or a tricyclic. The antidepressant-treated women had blood samples drawn at all prenatal interviews for determination of serum levels of the drug, to confirm that SSRI exposure actually occurred (
7). The diagnosis of major depressive disorder was made according to the Structured Clinical Interview for DSM-IV (SCID). To track depression, we adapted the timeline technique (
29) to chart mood episodes across time. For interviews after intake, the Longitudinal Interval Follow-Up Evaluation (LIFE) (
30) was used in conjunction with the SCID to assess for change in depression diagnostic status. Additional exposures to prescribed drugs, over-the-counter medications, environmental agents, alcohol, or smoking also were recorded at each assessment. Urine drug screens were obtained for all subjects at study enrollment. To differentiate the impact of SSRI from depression exposure, we evaluated three nonoverlapping groups of subjects according to their pregnancy exposures:
1.
No SSRI, no depression (N=97). These women had no exposure to any antidepressant or to major depressive disorder.
2.
SSRI (N=46). The majority (N=30) of women with SSRI exposure were treated continuously throughout gestation. This group also included women exposed to SSRIs in the first and/or second trimester but not the third (N=10) and women exposed in the second and/or third trimester but not the first (N=6).
3.
Depression, no SSRI (N=31). These women experienced full syndromal major depressive disorder at some point during pregnancy and received no antidepressant treatment. Of these women, eight were continuously depressed throughout pregnancy, 13 were depressed in the first and/or second trimester but not the third, and 10 were depressed in the second and/or third trimester but not the first. This group was included to evaluate the effects of active antenatal depression on pregnancy and fetal outcomes.
Growth Assessments
Infant weight, length, and head circumference were measured by a physician or physician’s assistant who was blind to depression and SSRI exposure status both during pregnancy and postpartum. Weight was measured on a standard digital scale accurate to 1 g. Length (in centimeters) was measured at each time point by stretching the infant from the crown of the head to the heel on a pediatric examination table with a built-in ruler. Head circumference (in centimeters) was measured with a pull-through tape to 1 mm; the tape was firmly tightened around the maximum circumference of the head. Although the target times for study visits were 2, 12, 26, and 52 weeks postpartum, some variability in time of measurement occurred, as anticipated, and the growth data were analyzed according to the exact week after birth that the infants were evaluated. Preterm infants were seen at the age-corrected visit; that is, the number of weeks less than 40 (full term) was added to the planned assessment target time and the data were plotted with this correction.
Statistical Methods
Descriptive statistics for the study group are reported as means and standard deviations for continuous variables and percentages for discrete variables. The comparison of subject characteristics across the three exposure groups was conducted with a chi-square test for discrete variables and an analysis of variance for continuous variables.
Mixed-effect regression models were used to assess the impact of antenatal exposure group on infant weight, length, and head circumference at 2 weeks and at 3, 6.5, and 12 months. All demographic and clinical characteristics that significantly differed among the three exposure groups were included in the modeling procedures. To include the potential impact of continuing maternal depression on growth, the models also included the presence or absence of the diagnosis of depression at each postpartum assessment time. Indeed, exposure to depression at each follow-up time point was found to be significantly associated with antenatal exposure (independent of the effect of time); therefore, it was included as a covariate in the analyses. Main fixed effects were included for antenatal exposure group, time, and baseline characteristics that differed among the three exposure groups. A two-way fixed-effect interaction was included for the interaction of exposure group and prepregnancy BMI and the interaction of exposure group and time. Random effects were included for intercept and slope. A p value of 0.05 was used to designate statistical significance, and the Bonferroni correction was used for multiple comparisons (set at p<0.0167).
Results
Of the 238 pregnant women enrolled in the parent study (
7), 71 (29.8%) were classified as having SSRI exposure, 36 (15.1%) had depression exposure (no SSRI), and 131 (55.0%) had neither depression nor SSRI exposure (comparison subjects). Of these original study subjects, 46 (64.8%) of the women exposed to SSRIs, 31 (86.1%) of the women with depression, and 97 (74.0%) of the comparison subjects had sufficient longitudinal data to be included in this analysis. The inclusion rate did not significantly differ across the three groups (p=0.14).
The comparison of the characteristics determined at intake across the three exposure groups is presented in
Table 1. However, many significant differences in the women’s demographic and clinical characteristics were observed even after Bonferroni correction. The only three variables that did not differ across groups were age, smoking, and alcohol intake during pregnancy. With respect to race, more minority women were present in the depression group, and the post hoc pairwise comparisons yielded a significant difference between the depression and comparison groups and between the depression and SSRI groups. The SSRI and comparison groups had similar high educational status, and both significantly differed from the less well-educated depression group. Women in the comparison group were significantly more likely to be employed than were those in the SSRI and depression groups. Although marital status differed, with more women in the depression group being single, the post hoc comparisons were not significant after Bonferroni correction. There was a significant difference in the average prepregnancy BMI, with women in the SSRI group being significantly heavier than the comparison group in post hoc analyses. Women in the SSRI and depression groups had more children than did the comparison subjects, without significant differences after adjustment for multiple comparisons.
Maternal clinical measures included the presence of a comorbid lifetime anxiety disorder, which differed across groups (
Table 1). More than 40% of women in both the depression and SSRI groups had lifetime diagnoses of anxiety disorders, compared with 23.7% of the comparison subjects. However, the post hoc comparison was significant only for SSRI versus no exposure. The score on the SIGH-ADS for depressive symptom level at intake differed across groups, as expected. Both the depression and SSRI-treated groups significantly differed from the comparison group.
Table 2 displays demographic and clinical measures for the infants. The preterm birth rate was significantly different across the groups, with the post hoc comparison showing a higher rate in the SSRI group than in the comparison group and no significant difference between the depression and SSRI groups or between the depression and comparison groups. Infant sex also differed across groups, with significantly fewer males in the SSRI group than in the comparison group. Unlike the subjects from the parent study (
7), this subgroup of women had infants that significantly differed with respect to birth length, but not birth weight or head circumference, after correction for gestational age. The rate of breast-feeding did not differ across the three groups.
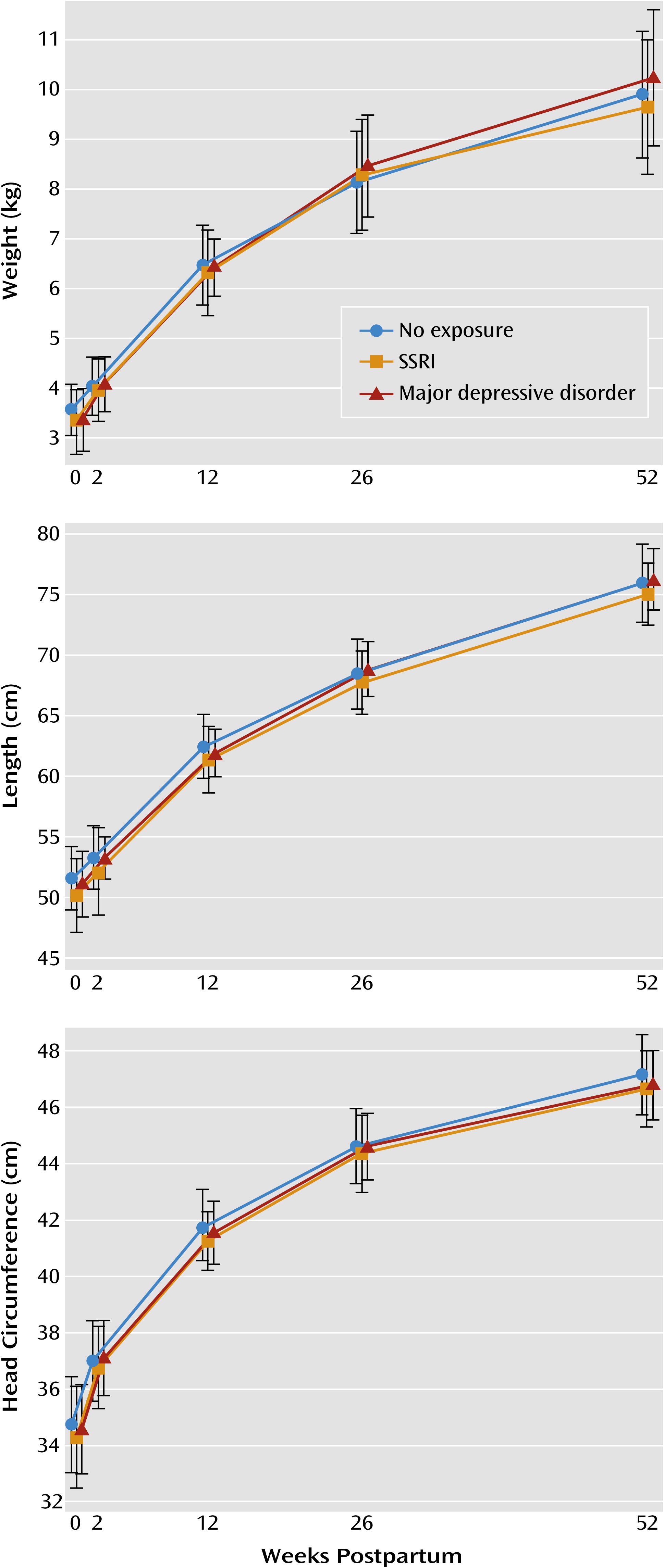
The longitudinal measurements of infant weight, length, and head circumference by group are presented in
Figure 1. No significant association between prenatal SSRI or depression exposure and growth in weight, length, or head circumference was observed. The unadjusted analysis revealed no association of prenatal exposure to weight (p=0.20), length (p=0.29), or head circumference (p=0.26). After we controlled for the characteristics that differed between exposure groups (race, education, employment, marital status, parity, presence of lifetime anxiety disorder, infant sex, and preterm birth) and included presence of depression at each postpartum time point, no significant association of exposure with weight (p=0.60), length (p=0.93), or head circumference (p=0.93) was observed. In addition, because maternal body weight affects aspects of infant growth, we evaluated the interaction of group and prepregnancy BMI, which was also nonsignificant, and no synergistic effect was identified for weight (p=0.87), length (p=0.79), or head circumference (p=0.97).
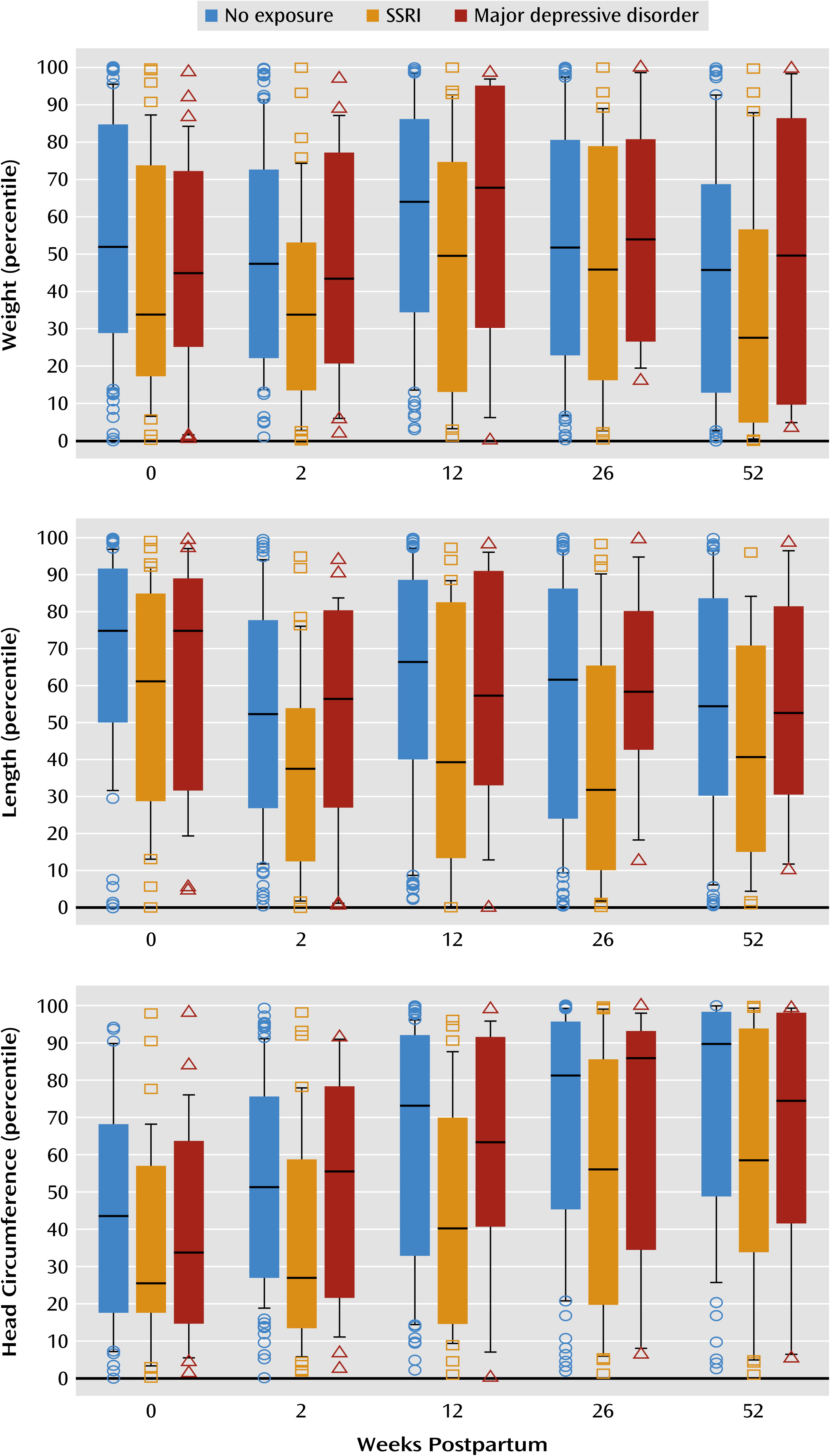
The values observed in our study group were compared with the population statistics from the Centers for Disease Control and Prevention (
www.cdc.gov/growthcharts/clinical_charts.htm) (
Figure 2). The mean length and weight measurements in all exposure groups were within the interquartile range (25th to 75th percentiles) of the general population of infants; therefore, the study group is reasonably similar to the general population. The mean head circumferences were within the interquartile range for all exposure groups with three exceptions: infants prenatally exposed to depression exceeded the 75th percentile at 26 weeks of age, and infants with no exposure exceeded the 75th percentile at 26 and 52 weeks of age.
Discussion
In this longitudinal observational study of in utero exposure to depression or SSRIs, we found no significant impact of either exposure on infant growth variables (weight, length, or head circumference) from birth through 12 months of age, i.e., infants with these exposures did not differ significantly from infants with no exposure. This investigation exemplifies the challenges of conducting observational reproductive outcome studies of pregnant women with diseases and the drugs used to treat them, a situation for which random assignment to treatment groups creates ethical dilemmas (
7). The demographic and clinical characteristics differed markedly across the depressed and nondepressed exposure groups, and these variables also may differentially influence reproductive outcomes. Likewise, the distribution of women with depression into SSRI-treated and unmedicated groups does not occur at random. Variables that differed across groups were included in the modeling procedures, but other, unidentified, unmeasured variables that differ across groups may also affect reproductive outcomes and always are a concern when interpreting data from observational studies.
Few studies of depression during pregnancy have considered anxiety comorbidity. The presence of an anxiety disorder in over 40% of the group with depression (with or without SSRI treatment), compared with only 24% in the comparison group without depression, is consistent with epidemiologic findings (
31). Anxiety is another exposure (for which we adjusted our models) that affects reproductive outcomes, such as risk for preterm birth, birth weight, and fetal and infant neurodevelopment (
32,
33).
The strengths of the study are inclusion of not only an SSRI-exposed but also a depression-exposed group of mothers, detailed exposure data collected prospectively, use of a urine drug screen to identify and exclude substance users, exclusion of women with antenatal exposure to FDA class D or X agents (which may be associated with increased risk for adverse pregnancy outcomes), blinded assessments of infant growth variables, and collection of growth data for infants across the first postpartum year. The major weakness is a relatively small study group, particularly for the mothers with depression. Between 65% and 86% of the original groups from the parent study contributed data. A larger group of subjects would have been preferable, particularly with the inclusion of a substantial number of variables that differed among exposure groups in the modeling procedures across time.
Further research to expand the number of subjects, which likely would require multisite studies, would allow subcategorization of the SSRI group based on the degree to which the treated woman achieved symptom reduction (such as responders or remitters), in order to evaluate whether the outcomes from effective SSRI treatment without the presence of depression are more favorable than those of women with untreated depression. This is the reason that pregnant women are treated with pharmacotherapy; that is, the anticipated overall benefit is greater than the risk (
23). The prescription of a drug for a pregnant woman results from the physician’s judgment that her health is best served by treatment of the disorder, yet our literature is largely focused on negative outcomes rather than potential positive effects on maternal disease and child outcomes (
34,
35). For example, Hunter et al. (
36) demonstrated that infants born to mothers with anxiety disorders had impaired P50 auditory gating (a marker of infant attentional processing). Their novel finding was that maternal antidepressant treatment during pregnancy improved sensory gating in the offspring.
Women who have SSRI exposure and continue to fulfill criteria for depression may be at the highest risk for adverse reproductive outcomes. Studies on a much larger scale also would provide the number of subjects required to evaluate reproductive outcomes related to individual SSRIs. The effect of SSRI dose (
3) or, more directly, maternal serum drug level or other measure of biological impact on reproductive outcomes is also needed to drive the process of risk-benefit decision making to a new level of sophistication (
23).
Acknowledgments
The authors thank Ms. Emily Pinheiro for incorporating the references.