A growing body of evidence demonstrates the presence of epigenetic alterations in the brains of suicide completers. Relative to comparison populations, suicide completers show lower expression of the astrocytic variant of the tyrosine kinase receptor B (TrkB-T1) gene in association with promoter hypermethylation in the prefrontal cortex (
7), site-specific hypermethylation in the promoter of the brain-derived neurotrophic factor variant IV (BDNF-IV) associated with decreased expression in the Wernicke area (
8), and decreased expression of the GABA-ergic receptor alpha subunit (GABA
α) gene with hypermethylation in its promoter in the prefrontal cortex (
9). Interestingly, these associations were independent of the possible effect of psychopathology. More recently, hypermethylation in the promoter of the glucocorticoid receptor gene was associated with decreased expression of glucocorticoid receptor in the hippocampus of abused suicide completers (
10,
11). These findings are supported by studies in animal models of maternal and stress-induced depressive-like behaviors showing DNA methylation alterations in animal brains (
12).
Collectively, these data suggest that DNA-specific methylation patterns are associated with suicide, independently from psychopathology. To date, this hypothesis has been primarily investigated by candidate-gene studies. Our understanding of gene interactions, however, suggests that genes act in complex networks, and it therefore stands to reason that DNA methylation involved in complex pathophysiology leading to suicide is not limited to a few candidate genes. We report results from a comprehensive genome-wide screening of promoter DNA methylation modifications found in the hippocampus of suicide completers compared with nonpsychiatric sudden-death comparison subjects. The results support the hypothesis that promoter DNA methylation patterns are altered in a coordinated way, affecting gene expression throughout the genome.
Discussion
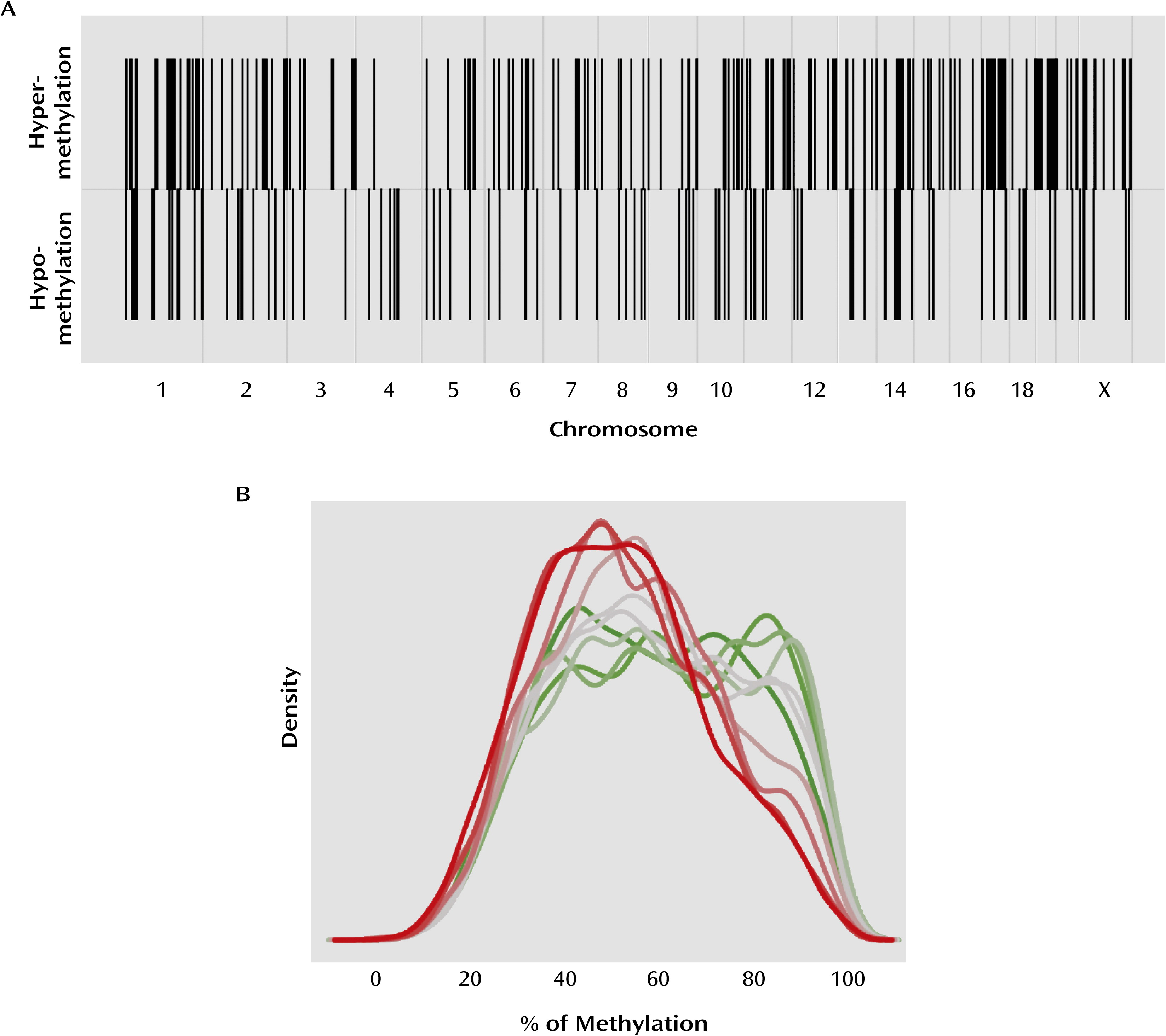
We examined DNA methylation patterns in the hippocampus of suicide completers and nonpsychiatric sudden-death comparison subjects at the proximal promoter regions of all known genes. In accordance with previous gene candidate studies (
12), our results suggest that promoter DNA methylation levels are significantly greater for several genes in suicide completers relative to comparison subjects. In addition, our results reveal the existence of a significant number of hypomethylated sequences in the promoters of suicide completers, suggesting that DNA methylation patterns are altered in the brains of suicide completers, with both hyper- and hypomethylation found in gene promoters across the genome.
DNA methylation is a major regulator of gene expression (
4). In agreement with previous findings in brain tissue (
12), our results show that overall in the genome, gene expression is negatively correlated with promoter methylation. This observation is also supported by our expression data on validated genes. Differentially methylated regions were found evenly distributed across the genome, an observation that has also been reported in the blood of patients with posttraumatic stress disorder and in brain tissue of psychotic and bipolar subjects (
22,
23), suggesting that epigenetic changes in psychiatric disorders may not be limited to a short list of specific gene candidates but may affect multiple functional gene networks in multiple chromosomal regions.
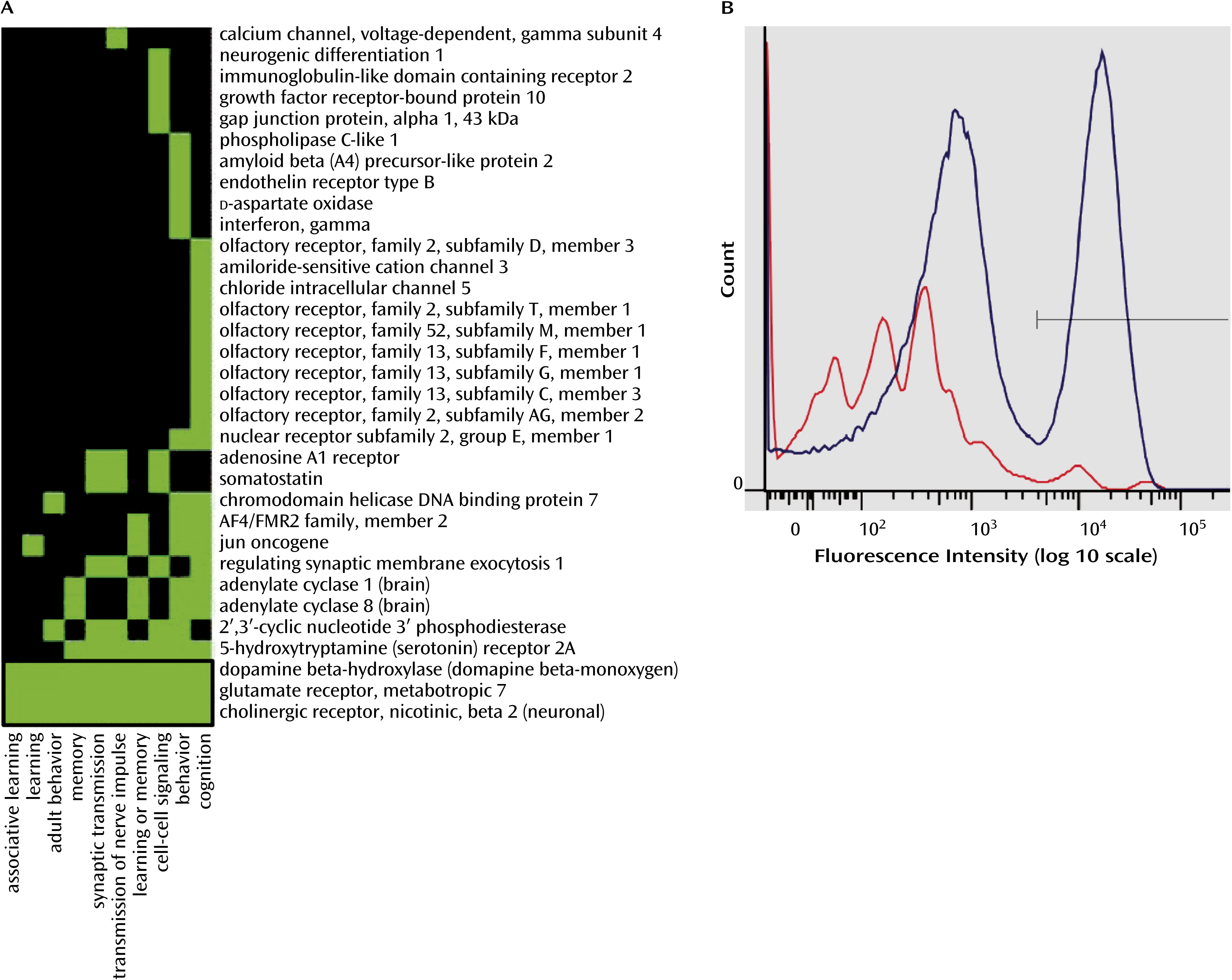
Suicide is a complex problem that most likely results from multiple pathological pathways (
24). Epigenetic alterations are expected to increase vulnerability to suicide by interfering with normal gene expression patterns, leading to neurobiological abnormalities associated with the development of specific emotional and behavioral phenotypes (
25,
26) as well as cognitive impairments (
27,
28). The biological functions identified by our ontological analyses, as the most significantly enriched in genes with differential promoter methylation, are related to behavioral and cognitive processes such as learning and memory. This implies that the epigenetic regulation of these processes in the brain may be altered in suicide completers, leading to the dysregulation of cognitive processes.
We targeted four genes known to be involved, whether directly or indirectly, in the regulation of the cellular processes of learning, memory, and behavior (
29–
33). NR2E1 encodes a brain-specific orphan nuclear receptor acting as a transcriptional repressor (
33), and GRM7 codes for the G-protein coupled metabotropic glutamate receptor subunit 7 (
34). CHRNB2 encodes a brain-specific subunit (β2) of the ligand-gated ionotropic nicotinic acetylcholine receptor family (
35,
36), and DBH codes for a catecholamine-synthetic membrane-bound enzyme responsible for the synthesis of norepinephrine (
37).
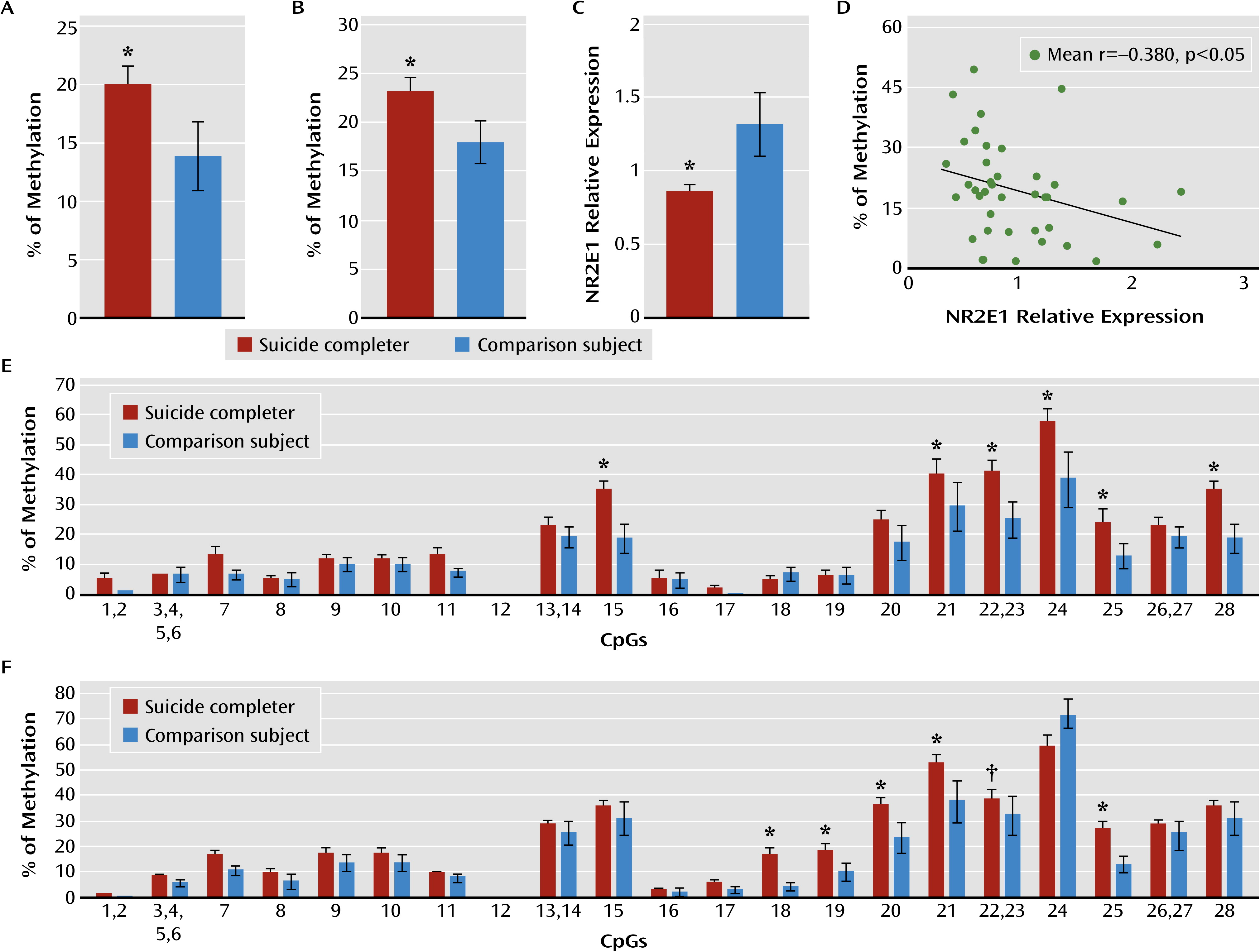
Our results revealed that both NR2E1 and GRM7 promoter methylation levels were associated with lower gene expression in the hippocampus of suicide completers. Interestingly, NR2E1 knockout mice exhibit severely aggressive and impulsive behaviors, blunted anxiety, fear conditioning, learning and memory deficits, and reduced mating (
32). Similarly, GRM7
−/− mice display a marked reduction in fear-mediated freezing responses (
38) and short-term working memory deficits in the four- and eight-arm maze task (
39). These behavioral phenotypes parallel many behavioral traits in humans that are regarded as risk factors for suicidal behavior. For instance, developmental trajectories characterized by high anxiety traits and externalizing behaviors (impulsivity, aggression) are predictive of suicide (
40). It is possible that changes in DNA methylation within promoters of a subset of genes regulating behavioral traits may lead to regulatory changes in the establishment of stable emotional and behavioral trajectories and associate with higher levels of anxiety, aggression, and impulsivity, which may in turn increase vulnerability to suicide.
Vulnerability to suicide has also been associated with several cognitive deficits (
28). Interestingly, in addition to NR2E1 and GRM7, we found differential promoter methylation in CHRNB2 and DBH, two genes that have been directly or indirectly associated with learning and memory formation by modulating long-term potentiation or long-term depression (
29,
31). Chronic, but not acute, pretreatment with nicotine reverses the effects of chronic psychosocial stress on long-term potentiation and long-term depression in rats (
41,
42). Consistently with the cognitive deficits observed in individuals with a past history of suicidal acts, these deficits seem to be in close relationship with the capacity to respond effectively to stressful situations. Indeed, individuals with altered hypothalamic-pituitary-adrenal axis reactivity fail to improve in retest of executive function (
43) and show more decision-making impairments (
44) following stress. Together, our results showing differential promoter DNA methylation associated with changes in the expression of genes involved in the cellular processes of learning and memory are consistent with current theoretical models of the neurobiology of suicidal behavior, and thus it is possible that these alterations are etiologically related to suicide (
45).
On the other hand, the role of DBH in the hippocampus may rather be indirect through its role in the synthesis of norepinephrine. Indeed, norepinephrine is suspected to enhance contextual fear memory and long-term potentiation in the hippocampus through phosphorylation of GluR1 subtypes, facilitating AMPA trafficking at synaptic sites (
46). However, our data show no difference in DBH expression between suicide completers and comparison subjects, suggesting that other mechanisms regulating expression, such as histone modifications, may be involved. More research is needed to elucidate the complex relationship between DNA methylation and histone modifications.
Concordant with their expression patterns, the hypermethylation in the promoter of NR2E1, CHRNB2, GRM7, and DBH was almost specific to the neuronal cell fraction. Indeed, CHRNB2, GRM7, and DBH are expressed only in neurons (
37,
47,
48), whereas NR2E1 expression is not restricted to neurons. Our data suggest that the hypermethylation found within the neuronal cell fraction may be responsible for the lower expression of NR2E1, GRM7, and CHRNB2 genes in the hippocampus of suicide completers.
However, we found significant hypomethylation in CHRNB2 and GRM7 promoters in the nonneuronal cell fractions, suggesting that the same genes could be differently affected in different cell types, with cellular consequences proper to each cell type. This is consistent with the fact that different tissues (
49) and cell types within the same tissue (
21,
50) exhibit different DNA methylation patterns. However, it is hard to speculate on the functional impact of these findings, especially given that these observations were made on a highly heterogeneous cell population composed of different nonneuronal cell types and that these genes are expressed primarily in neurons.
Our results are concordant with results we recently published on the epigenetic effects of early-life adversity in the brain (
51). While early-life adversity was also associated with a genome-wide epigenetic reprogramming, the differentially methylated genes observed in the two studies were different and are known to be involved in different functional pathways. Thus, the data in the present study expand our understanding of the role of epigenetic mechanisms in the brains of suicide completers and provide future avenues of investigation in the study of molecular processes in the brains of suicide completers.
The data we present here have been adjusted for covariates susceptible to influencing DNA methylation and expression (history of adversity, postmortem interval, substance use disorder comorbidity, and age). It is known, however, that medication alters DNA methylation levels both in the brain and in peripheral tissue (
52,
53). Although our analyses suggest that medication may not have significantly affected DNA methylation in our study, more research is needed to investigate the role of medication in DNA methylation in the human brain.
One important consideration regarding our study is the method used to isolate methylated DNA. MeDIP is a highly sensitive method for the enrichment of methylated genomic DNA (
15) that is not limited by sequence context of methylation-specific enzymes and does not require extensive bisulfite treatment. Although MeDIP enriches DNA sequences with both low and high CpG density, it is more sensitive to methylation in CpG-rich regions and CpG islands (
54). The extent of promoters’ CpG enrichment varies throughout the genome (
55), and CpG density within promoters has been associated with different DNA methylation levels. Generally, CpG-rich promoters have been associated with low DNA methylation levels, while low-CpG density promoters have been shown to be more methylated (
55,
56). Thus, it is possible that our results represent only a subset of even larger methylation changes taking place throughout the genome, given that some differences in gene promoters with low CpG density may not be well represented.
In addition, it has been suggested that the 5′ methylcytosine antibody may capture, to a certain extent, non-CpG methylation (
54), which has classically been reported in embryonic and induced pluripotent stem cells (
57–
59), but also recently in mouse brains (
60). It is unlikely, however, that this happened in our study. Indeed, our analyses were focused on gene promoters, and non-CpG DNA methylation has been reported mainly in gene bodies (
57). However, more research is needed to address this question.
In summary, our results show DNA methylation differences in gene promoter regions throughout the genome in the hippocampus associated with suicide. These changes in methylation levels are in genes involved in the regulation of behavioral and cognitive processes that have been shown to be altered in individuals with suicidal behaviors. Thus, our findings suggest that such mechanisms may, in individuals with particular behavioral, molecular, and cellular predispositions, increase susceptibility to suicide.