The “horse race” between the ultimate effectiveness of psychotropic medications versus psychological treatments, and the search for a decisive “winner,” is largely over. A sizable body of evidence now reveals an important role for both pharmacologic and psychological dimensions of care for the majority of patient conditions. And relatedly, it has become increasingly clear that simplistic factorial designs examining overall main effects and combined treatment strategies can only carry our field so far. The more appropriate and clinically relevant pursuit is to elucidate the manner in which complementary psychotropic and psychological strategies are to be best sequenced or integrated in ways that maximize sustained clinical response while also minimizing risks to patients, costs to payers, and burdens to overall systems of care. As we strive toward an era of evidence-based personalized medicine, the most revealing empirical question is not whether pharmacologic or psychological strategies are indicated, but rather when, under what circumstances, for whom, at what intensity, and in what sequence or combination such treatments best perform.
In the article by Wetherell et al. (
1), published concurrently with this editorial, the authors present the results of a clinical trial that begins to ask some of these increasingly important and revealing questions with specific regard to a highly understudied clinical arena: the treatment of older adults with generalized anxiety disorder. Characterized by persistent, uncontrollable, and debilitating worry, generalized anxiety disorder is associated with considerably reduced health-related quality of life (
2), poor physiological health, and increased health care utilization (
3,
4) and appears to be the most prevalent mental disorder affecting older adults. In smaller trials examining broad main effects of treatments, antidepressant medications and, to a lesser extent, cognitive-behavioral therapy (CBT) have shown clinical utility in managing late-life generalized anxiety disorder, but much remains to be learned about how such findings can inform sequential treatment courses that typify practice and attend to the shifting clinical demands of patients across time.
Using a multisite randomized placebo-controlled sequential treatment design, Wetherell et al. were able to examine the merits of augmenting initial response to 12 weeks of antidepressant medication with 16 subsequent weeks of continued antidepressant medication or 16 weeks of antidepressant medication plus CBT. After completing this augmentation phase, participants were randomly assigned to receive either 28 maintenance weeks of continued antidepressant medication or placebo. The study found that participants who received CBT during the augmentation phase were roughly three times more likely to show meaningful reductions in worry relative to those who simply received continued antidepressant medication, and those who received continuous medication across the entire maintenance phase (with or without CBT) were highly protected against subsequent relapse.
There is much to commend in this study. The authors are some of the most senior and experienced experts on later-life generalized anxiety disorder, and the CBT protocol evaluated is a particularly well-put-together state-of-the-art intervention. Although antidepressant medication has shown utility in the acute treatment of late-life generalized anxiety disorder (
5), extended follow-up evaluations have been rare, and practitioners are regrettably left with limited evidence with which to inform clinical efforts to maintain antidepressant treatment response and prevent relapse. Partly as a consequence, we have in recent years seen a progressive expansion in the use of antipsychotic medications to manage generalized anxiety disorder and other anxiety disorders across the lifespan (
6), often in an effort to augment antidepressant medication effects despite limited evidence supporting the safety and effectiveness of such off-label practices. Antipsychotic medications are particularly concerning with regard to older populations because of their elevated associations with falls and fractures, cognitive worsening, cardiac arrhythmia, cerebrovascular events, and mortality (
7). Hence, these data supporting the highly positive role of CBT for augmenting response to antidepressant medication are highly welcome.
Notably, although the design of Wetherell et al. improves on previous work in this area by examining patient outcomes across multiple phases (i.e., augmentation, maintenance) and evaluating treatment sequences in a randomized controlled manner, this study is limited in the extent to which it can inform clinical practice because only patients who responded to initial antidepressant medication were included in the randomization. Few clinicians would prescribe a time-consuming and expensive CBT course to patients who have already evidenced a positive response to antidepressant medication, despite the outcomes of this study. Some essential questions remain: What do we do with patients who do
not respond to initial antidepressant regimens, and how should CBT be incorporated into dynamic treatment regimens in order to optimize response for such individuals? Moreover, this study does not address the optimal course of treatment for individuals receiving CBT as a first step of care, which could entail its own concerted multisite effort (for examples of recent multisite efforts examining treatment sequences for positive and negative responders to initial CBT treatment for panic disorder, see reference
8 and unpublished 2012 data of L.A. Payne et al.). Given accumulated evidence supporting roles for both psychotropic medications and psychological treatments across patient populations, further work is now needed to build on achievements such as the Wetherell et al. study and provide empirical guidance for optimally tailoring anxiety disorder treatment sequences in response to dynamically unfolding patient outcomes.
Although data from the revealing Wetherell et al. design can only speak to the care of patients who are fortunate to show initial antidepressant treatment response, recent advances in intervention science offer innovative methodological and design options for the systematic evaluation of treatment sequences that flexibly adapt to patients’ fluctuating responses over time (i.e., adaptive treatment regimens). Specifically, over the past decade, we have seen the development, refinement, articulation, and increased use of sequential multiple assignment randomized trials (SMARTs) (
9,
10) to yield high-quality data with which to develop evidence-based adaptive treatment regimens that differentially incorporate the benefits of medication and psychological components of care across critical points in the course of treatment. Similar to the study conducted by Wetherell et al., a SMART includes multiple intervention stages, but as participants move through the intervention stages, their randomization options at critical decision points are typically determined by their performance or response at that point.
The design of a SMART improves on traditional factorial models focused exclusively on broad main effects of monotherapies and combined treatments across a single treatment phase, and it instead recognizes the true multiphase nature of the treatment process for the majority of patients in clinical practice.
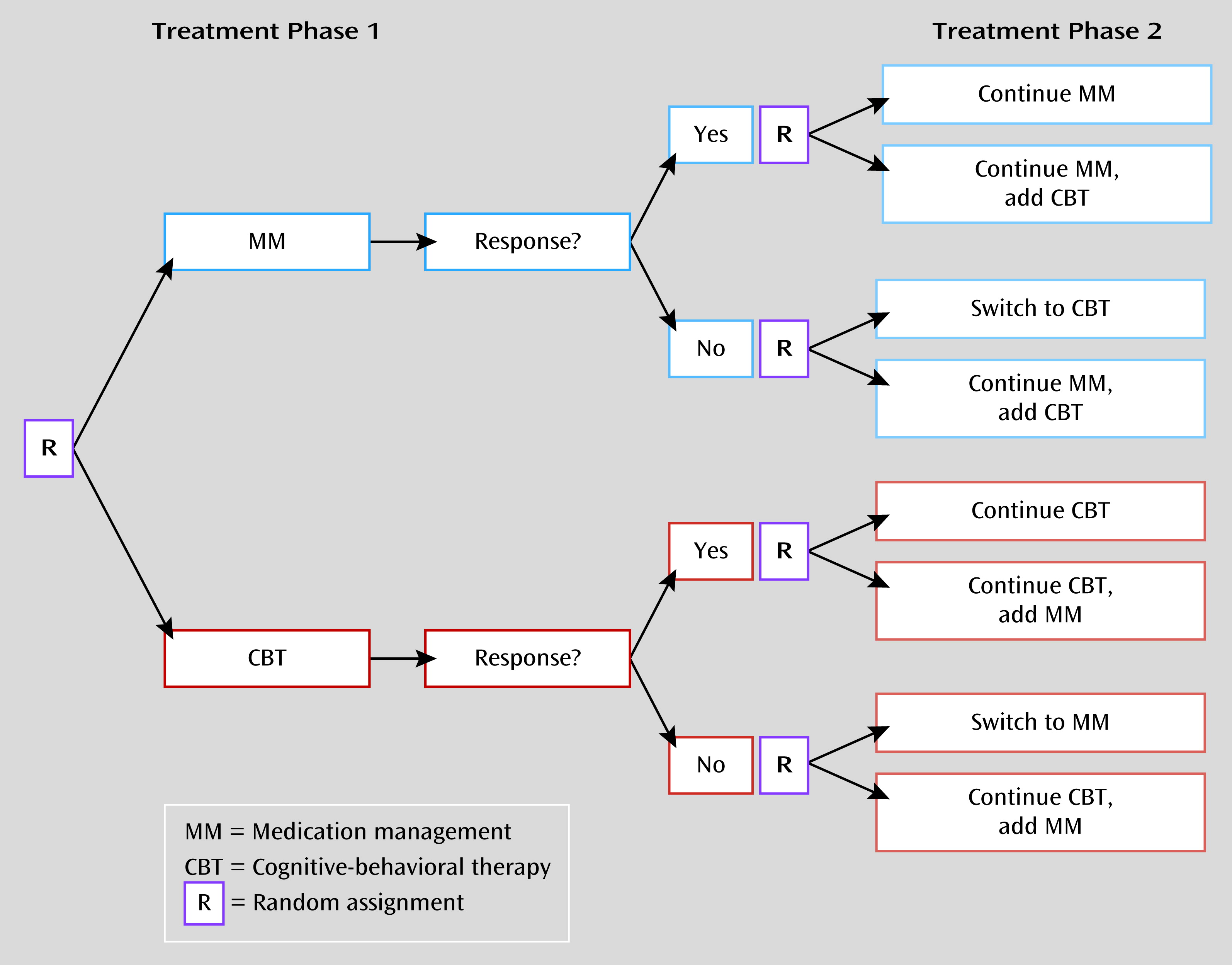
Figure 1 presents a generic SMART design, although SMARTs can certainly become much more complex, and accordingly can require very large sample sizes, depending on the number of interventions, treatment intensities, and treatment phases being evaluated. The design illustrated in
Figure 1 yields data to meaningfully inform eight distinct adaptive treatment regimens, and this single design can efficiently inform sequenced treatment decisions for patients demonstrating a range of clinical responses to different sets of initial treatments. Despite the adaptive nature of participants’ individual interventions across study treatment, the randomization procedures of a SMART at critical decision points allow causal conclusions to be drawn (
11).
Although the notion of personalized medicine is nothing new in mental health care, advances in intervention science, such as the SMART, and accompanying data-analytic advances now afford rigorous experimental methods with which to meaningfully inform the development of evidence-based adaptive intervention regimens. The SMART offers a hybrid of sorts between the predominant nomothetic groups-based (factorial) design strategy (
12) that is commonly used to inform policy decisions and the more idiographic single-case experimental designs (
13) used to understand individualized changes. In recent years, we have seen the completion of several revealing multisite trials incorporating key elements of SMART design into their experimental methodology for a number of the most interfering clinical conditions (e.g., the Clinical Antipsychotic Trials of Intervention Effectiveness project and the Sequenced Treatment Alternatives to Relieve Depression study), but regrettably we have not yet seen this sort of large-scale evaluation—in which patient responses at critical points in treatment determine subsequent randomization options that allow causal conclusions—in the study of anxiety disorder treatments, nor are we likely to soon, in view of current funding priorities. The closest such evaluation (
14) used a preferential treatment design in which those in the experimental condition were able to choose whether to receive CBT, medication, or both, thus limiting causal conclusions. This tremendous gap in our intervention sciences leaves us substantially underequipped to use experimental evidence to optimally inform the clinical management of the most prevalent and collectively impairing class of disorders affecting the general population.