Depressive episodes represent the most common symptomatic state associated with bipolar disorder (
1), resulting in greater impairment in work, family, and social functioning than episodes of mania (
2,
3). Compared with major depressive disorder, bipolar depressive episodes impose a greater burden on patients, caregivers, and society (
3–
5) and are associated with a higher risk of suicide (
6), substantially more lost workdays (
7), and more direct and indirect costs (
8).
Despite the high prevalence of depressive symptoms in patients with bipolar disorder, there are few evidence-based treatments for bipolar depression, and available guidelines reflect greater uncertainty regarding consensus treatment algorithms compared with both mania and unipolar depression. In the United States and in some other countries, quetiapine (immediate- and extended-release formulations [
9]) and the olanzapine-fluoxetine combination (
10) are approved for the treatment of acute bipolar depression. Lithium and valproate have demonstrated antimanic efficacy in bipolar disorder and are considered to be mood stabilizers but are markedly less effective in treating the depressive phase of the illness (
11). Although a variety of agents are added to mood stabilizers to treat depressive symptoms, very little evidence exists to guide treatment in the adjunctive setting. In particular, no positive studies showing efficacy of an atypical antipsychotic agent added to a mood stabilizer have been reported to date (
12). Although standard antidepressants are commonly used as adjunctive therapy (
13), evidence for efficacy in bipolar populations is lacking, and concerns exist regarding their potential to induce manic switching and reduce cycle length (
14,
15). Therefore, there is a significant need for adjunctive agents that have demonstrated efficacy when combined with mood stabilizers in patients with bipolar depression.
Lurasidone is a novel atypical antipsychotic with high affinity for D
2, 5-HT
2A, and 5-HT
7 receptors (antagonist), moderate affinity for 5-HT
1A receptors (partial agonist), and no appreciable affinity for H
1 and M
1 receptors (
16). In previous trials in schizophrenia, treatment with lurasidone has been associated with minimal effects on weight, lipids, or measures of glycemic control (
17). Lurasidone has been approved by the U.S. Food and Drug Administration for the treatment of bipolar I depression as monotherapy and as adjunctive therapy with lithium or valproate (
18). The primary objective of this placebo-controlled phase 3 study was to evaluate the efficacy of lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression.
Method
Patients
This international study enrolled outpatients 18–75 years of age diagnosed with bipolar I disorder who were experiencing a major depressive episode (DSM-IV-TR criteria, ≥4 weeks and <12 months in duration), with or without rapid cycling, without psychotic features, and with a history of at least one lifetime bipolar manic or mixed manic episode. Diagnosis was confirmed by the Mini-International Neuropsychiatric Interview (
19) and the Bipolarity Index (
20). A Montgomery-Åsberg Depression Rating Scale (MADRS [
21]) score ≥20 and a Young Mania Rating Scale (YMRS) score ≤12 were required at both screening and baseline. The investigator confirmed that the patient had not adequately responded to a minimum 28-day trial with doses of either lithium or valproate, based on information from a treating health care professional or a reliable informant. At screening, serum levels for lithium and valproate, respectively, were required to be 0.6–1.2 mEq/liter and 50–125 µg/ml.
Patients were excluded if they demonstrated a decrease of ≥25% in MADRS total score between screening and baseline; scored ≥4 on MADRS item 10 (suicidal thoughts) at screening or baseline; were judged to be at imminent risk of suicide or injury to self or others; had been hospitalized for a manic or mixed episode within the 60 days prior to randomization; had received treatment with antidepressants within 3 days, fluoxetine within 28 days, a monoamine oxidase inhibitor within 21 days of randomization, or clozapine within 120 days of randomization; had an acute or unstable medical condition; had a history of alcohol or substance abuse (past 3 months) or dependence (12 months); or had a history of nonresponse to an adequate (6-week) trial of three or more antidepressants (with or without mood stabilizers) during the current depressive episode.
The study was approved by an institutional review board at each investigational site and was conducted in accordance with the International Conference on Harmonisation Good Clinical Practices guidelines and with the ethical principles of the Declaration of Helsinki. All patients who entered the study reviewed and signed an informed consent document explaining study procedures and potential risks before study entry. An independent data and safety monitoring board reviewed and monitored patient data throughout the study.
Study Design
A total of 348 patients were randomly assigned at 58 sites in Europe (N=132), North America (N=114), Asia (N=84), and Africa (N=18). This study was conducted between May 2009 and January 2012.
Patients underwent stratified randomization, based on treatment with either lithium or valproate, to either adjunctive lurasidone 20–120 mg/day or placebo in a 1:1 ratio via an interactive voice response system. Study medication was provided in blister packs as either lurasidone 20 mg or 40 mg, or identically matched placebo tablets. Lurasidone treatment was initiated at 20 mg/day on days 1 to 3, increased to 40 mg/day on days 4 to 6, and then 60 mg/day on day 7. After the first week, lurasidone could be adjusted within the dosage range of 20–120 mg/day at weekly intervals, in 20-mg increments or decrements, based on investigator judgment. Lurasidone (or placebo) was taken once daily in the evening, with a meal or within 30 minutes after eating. The dose of mood stabilizer was adjusted to maintain a serum level in the range of 0.6–1.2 mEq/liter for lithium or 50–125 μg/ml for valproate throughout the study.
Concomitant Medications
Treatment with anticholinergic agents, propranolol, or amantidine, was permitted as needed for movement disorders. Lorazepam, temazepam, or zolpidem (or their equivalent) were permitted during screening and for weeks 1 to 3 as needed for anxiety or insomnia, but not within 8 hours prior to any psychiatric assessments.
Efficacy Assessments
Efficacy assessments were obtained at baseline and weekly intervals. The primary efficacy endpoint was the mean change from baseline to week 6 in MADRS total score. The MADRS, a 10-item scale with a total score that ranges from 0–60, was administered at each study visit by qualified centralized raters (via videoconferencing; available in the United States only) and by qualified site-based raters in all other countries. The key secondary efficacy endpoint was the mean change from baseline to week 6 in the depression severity score on the Clinical Global Impressions scale for use in bipolar illness (CGI-BP) assessment, which rates severity of depression on a 7-point scale.
Additional secondary efficacy assessments included the 16-item Quick Inventory of Depressive Symptomatology, self-rated version (
22), the Hamilton Anxiety Rating Scale (HAM-A [
23]), the Sheehan Disability Scale (
24), and the Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form (
25).
Safety and Tolerability Evaluations
Safety and tolerability were assessed by the incidence and severity of adverse events during the study. Movement disorders were assessed by the Simpson-Angus Scale, the Abnormal Involuntary Movement Scale, and the Barnes Akathisia Rating Scale. Additional safety evaluations included vital signs, laboratory tests, 12-lead ECG, and physical examination. Treatment-emergent mania was defined a priori as a Young Mania Rating Scale (YMRS [
26]) score ≥16 on any two consecutive visits, or at the final assessment, or an adverse event of mania or hypomania. Suicidal ideation and behavior were assessed using the Columbia Suicide Severity Rating Scale.
Statistical Analysis
The intent-to-treat population consisted of randomly assigned patients who received at least one dose of study medication and had at least one postbaseline efficacy assessment. The primary (MADRS) and key secondary (CGI-BP depression severity) efficacy endpoints were assessed using a mixed model for repeated-measures analysis including treatment, visit, pooled center, stratification variable (lithium or valproate), baseline score, and a treatment-by-visit interaction term, using an unstructured covariance matrix for within-patient correlation. To preserve the type I error rate, a sequential testing procedure was employed, with the primary efficacy variable tested first; only if the result was statistically significant was the key secondary variable (CGI-BP depression severity) tested.
Change from baseline in secondary efficacy measures was evaluated using an analysis of covariance (ANCOVA) model with fixed effects for treatment, pooled center, stratification variable (lithium or valproate), and baseline score as a covariate; secondary measures were not corrected for multiplicity. Core depressive symptoms were evaluated using the MADRS-6 subscale (
27). The proportions of responders (≥50% reduction from baseline in MADRS total) and remitters (MADRS total ≤12) were compared between the lurasidone and placebo treatment groups using logistic regression. Cohen’s d effect sizes were calculated for the primary outcome as the difference in the change score divided by the pooled standard deviation. The number needed to treat to attain an additional responder was derived for the lurasidone group as follows: number needed to treat=1/(lurasidone response rate−placebo response rate).
The safety population included all patients who were randomly assigned and received at least one dose of study medication. Descriptive statistics were used for safety variables including adverse events, vital signs, and laboratory results. In addition, rank ANCOVA was used to analyze weight, cholesterol, triglycerides, glucose, and insulin to compare changes from baseline among treatment groups. Change from baseline to last-observation-carried-forward endpoint in the Simpson-Angus Scale, the Abnormal Involuntary Movement Scale, and the Barnes Akathisia Rating Scale scores were analyzed using an analysis of covariance (ANCOVA) model with fixed effects for treatment, pooled center, and baseline as a covariate. Sample size was determined based on a two-sample t test, and was powered at 90% to detect a 3.25-point difference in MADRS change scores, with a pooled standard deviation of 9. The estimated sample size of 340 included an additional 16 patients (5%) based on expected early attrition (patients randomly assigned but without any postbaseline efficacy measures).
Results
Baseline Characteristics and Patient Disposition
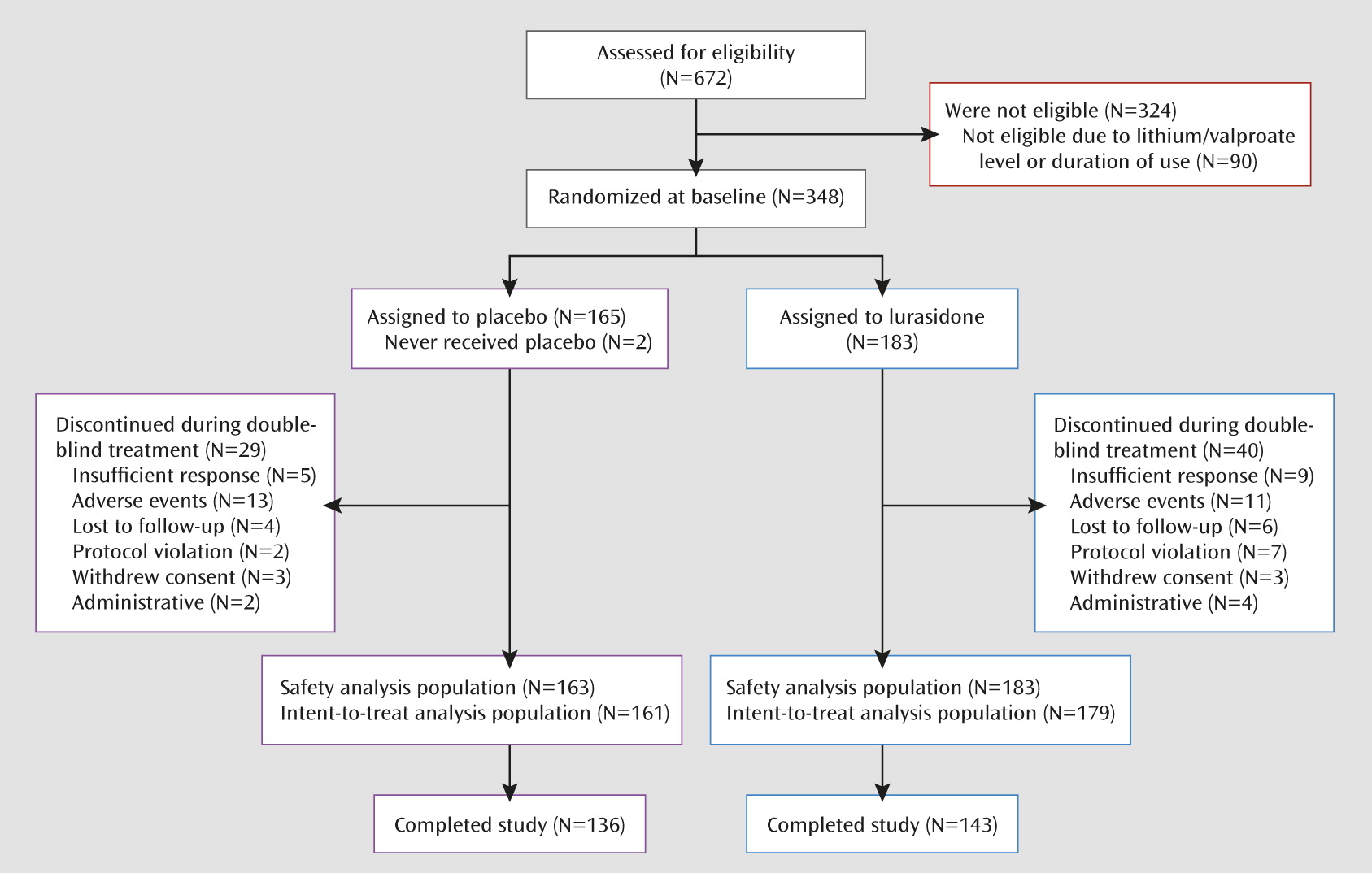
A total of 672 patients were screened, of whom 348 (51.8%) were randomly assigned to 6 weeks of double-blind treatment (
Figure 1). Baseline demographic and clinical characteristics were similar for the two treatment groups (
Table 1). Study completion rates were 78.1% for lurasidone and 82.4% for placebo (
Figure 1).
The proportion of patients receiving lithium or valproate at study entry was approximately 50% for each agent (
Table 1). Lithium concentrations were similar in the lurasidone and placebo groups, respectively, at baseline (0.72 and 0.71 mEq/liter) and week 6 endpoint (0.74 and 0.67 mEq/liter). Valproate concentrations were also similar in the lurasidone and placebo groups, respectively, at baseline (75.0 and 72.0 μg/ml) and at week 6 endpoint (72.4 and 71.1 μg/ml). The mean baseline doses of lithium in the lurasidone and placebo groups were 897.2 mg/day and 947.3 mg/day, respectively; the mean baseline doses of valproate were 1058.3 mg/day and 1117.8 mg/day, respectively.
The mean daily dose of lurasidone during the study was 66.3 mg and the mean modal daily dose was 75.2 mg. After completion of fixed titration to a lurasidone daily dose of 60 mg on day 7, 63.4% of patients increased to a dosage of 80 mg/day, 37.1% increased to a dose of 100 mg/day, and 18.3% increased to a dosage of 120 mg/day at some point during the remaining 5 weeks of study treatment.
Efficacy
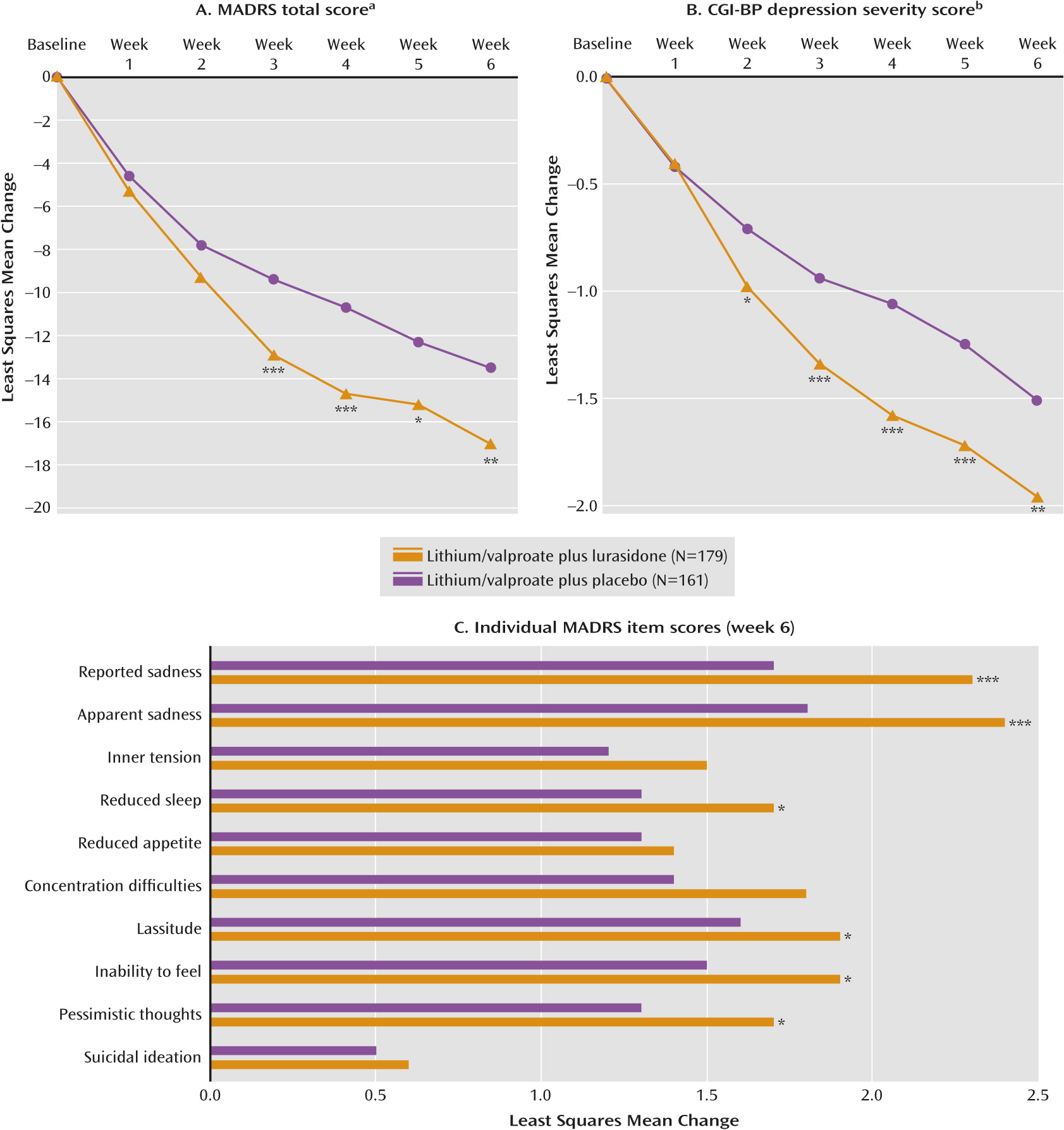
The least squares mean change from baseline to week 6 in MADRS total score was significantly greater for the lurasidone group compared with the placebo group (−17.1 versus −13.5; p=0.005 [effect size=0.34]) (
Figure 2A). Least squares mean change from baseline to week 6 in the CGI-BP depression severity score was significantly greater for the lurasidone group compared with the placebo group (−1.96 versus −1.51; p=0.003 [effect size=0.36]) (
Figure 2B). Statistical superiority versus placebo for the MADRS and CGI-BP depression severity score was observed starting at week 3 and week 2, respectively, and was maintained at all subsequent study visits for both outcomes.
Treatment effects associated with adjunctive lithium and valproate were comparable. Specifically, effect sizes at week 6 endpoint on the MADRS were 0.36 for lurasidone adjunctive with lithium and 0.32 for lurasidone adjunctive with valproate; effect sizes on the CGI-BP depression severity score were 0.33 for lurasidone adjunctive with lithium and 0.39 for lurasidone adjunctive with valproate.
There was a statistically significant reduction from baseline to week 6 in core depressive symptoms (MADRS-6 subscale score) in the lurasidone group compared with the placebo group (−11.6 versus −9.1; p=0.003). Treatment with lurasidone was associated with greater endpoint improvement compared with placebo on each of the 10 MADRS items, with a significant difference achieved on the following items: apparent sadness, reported sadness, reduced sleep, lassitude, inability to feel, and pessimistic thoughts (
Figure 2C).
A significantly greater proportion of patients met a priori response criteria after 6 weeks of treatment with lurasidone compared with placebo (57% versus 42%; p=0.008 [number needed to treat=7]). Median time to response was significantly shorter for the lurasidone group compared with placebo (28 versus 42 days; log-rank p<0.001). The proportion of patients achieving remission at endpoint was significantly greater in the lurasidone group compared with placebo (50% versus 35%; p=0.008 [number needed to treat=7]). The median time to remission was significantly shorter for the lurasidone group compared with placebo (35 versus 43 days, p=0.001).
No significant treatment interactions by gender, race, ethnicity, or age were observed for either the MADRS total score or the CGI-BP depression severity score, based on ANCOVA analyses. Least squares mean changes in scores from baseline to endpoint (lurasidone versus placebo) for secondary efficacy assessments were as follows: the Quick Inventory of Depressive Symptomatology (−8.1 versus −5.9; p<0.001); the Hamilton anxiety scale (−8.0 versus −6.0; p=0.003); the Quality of Life, Enjoyment, and Satisfaction Questionnaire–Short Form (+22.2 versus +15.9; p=0.003); and the Sheehan Disability Scale (−9.5 versus−7.0; p=0.012).
Safety
Overall, adverse events reported with an incidence ≥5% in the lurasidone group versus placebo were nausea, somnolence, tremor, akathisia, and insomnia (
Table 2). The majority of adverse events were considered mild or moderate. The incidence of serious adverse events was low and similar in the lurasidone (1.1%) and placebo (1.2%) groups. No deaths occurred during the study. The proportion of patients who discontinued due to adverse events was similar for lurasidone (6.0%) and placebo (7.9%) (
Figure 1).
The incidence of protocol-defined treatment-emergent mania was similar for the lurasidone group (1.1%, N=2) compared with the placebo group (1.2%, N=2). Baseline YMRS scores were low for both treatment groups (
Table 1) and showed a small decrease at endpoint (−0.7 versus −0.9; n.s.)
The proportion of patients with treatment-emergent suicidal ideation, per the Columbia Suicide Severity Rating Scale, was 8.9% in the lurasidone group and 5.6% in the placebo group. There were no instances of suicidal behavior or completed suicide in either treatment group during the study.
The incidence of extrapyramidal symptom-related adverse events was 15.3% in the lurasidone group and 9.8% in the placebo group (
Table 2); 11% of the lurasidone group and 4% of the placebo group received treatment with anticholinergic medication for acute extrapyramidal symptoms. Treatment with adjunctive lurasidone was associated with a small but significantly greater endpoint change compared with placebo in the Barnes Akathisia Rating Scale score global score (0.1 versus 0.0; p=0.009), and the Simpson-Angus Scale score (0.03 versus 0.01; p=0.018), but no difference for the Abnormal Involuntary Movement Scale total score (both groups, 0.0).
Mean endpoint change in weight was not significantly different between the lurasidone and placebo groups (0.2 kg versus 0.1 kg). The proportion of patients with ≥7% increase in weight at study endpoint was 3.1% in the lurasidone group and <1% in the placebo group. Mean endpoint change in waist circumference was also similar between the lurasidone and placebo groups (0.2 cm versus 0.4 cm).
There were no notable differences in laboratory measures, vital signs, or ECG assessments between treatment groups (
Table 3). The mean change from baseline to endpoint in the QTcF interval was 1.0 ms in the lurasidone group and 1.2 ms in the placebo group. One patient in the lurasidone group had a postbaseline change QTcF of ≥60 ms; no patient in either treatment group had a QTcF ≥500 ms.
Discussion
In this placebo-controlled 6-week study, flexibly dosed, once-daily lurasidone (20–120 mg) was effective and well tolerated in patients with bipolar depression when added to stable dosages of lithium or valproate. To our knowledge, this is the first large-scale, randomized, placebo-controlled trial to demonstrate efficacy of any medication adjunctive to mood stabilizers for the acute treatment of bipolar depression (
12), the most common and disabling phase of the illness (
1–
8). In particular, no positive controlled studies have been published to date regarding the use of atypical antipsychotic medications as adjunctive therapy for patients with bipolar depression.
Significantly greater improvement in the MADRS in favor of lurasidone versus placebo was observed from week 3 through week 6, yielding a clinically meaningful between-group effect size at endpoint (−17.1 versus −13.5; effect size, 0.34). The magnitude of improvement on placebo in the present study (−13.5) was somewhat higher than the improvement (−10.3 to −12.6) reported in previously published monotherapy trials of atypical antipsychotics in bipolar depression (
28) and in the lurasidone monotherapy trial (
29); this may be partially attributable to concurrent treatment with lithium or valproate.
The MADRS findings in this study were supported by significantly greater endpoint improvement in the CGI-BP depression severity score, the key secondary endpoint which assessed global severity of depressive symptoms. In addition, treatment with lurasidone was associated with significant reduction in anxiety symptoms and significant improvement in patient-rated functional impairment and quality of life.
While the flexible-dose design limited our ability to determine a dose-response relationship for lurasidone in this study, we note that a substantial proportion of patients (63.4%) received dosages of 80 mg/day or higher at some point during the 6 weeks of study treatment. The mean dose of lurasidone and the dose escalation observed in this study were likely influenced by the required fixed-dose titration to 60 mg/day by day 7.
The efficacy of atypical antipsychotic agents as monotherapy in bipolar depression has been demonstrated for quetiapine (
9), olanzapine (
10,
30), and the olanzapine-fluoxetine combination (
10,
31), but monotherapy studies of aripiprazole and monotherapy and adjunctive studies of ziprasidone have yielded negative results (
12,
32,
33). This variation in efficacy reported for atypical antipsychotic agents suggests that antidepressant activity is not consistently present across the class (
34). In addition to high affinity for dopamine D
2 and serotonin 5-HT
2A receptors (antagonist), lurasidone has high affinity for 5-HT
7 receptors (antagonist), and moderate affinity for serotonin 5-HT
1A receptors (partial agonist [
16]). Lurasidone has demonstrated significant improvement in both acute and chronic animal models of depression (
35). Antagonist activity at 5-HT
7 receptors has been reported to be associated with antidepressant effects in animal behavioral models of depression (
35), and the absence of antidepressant activity in knockout mice indicates that this effect requires the presence of functional 5-HT
7 receptors (
35). Taken together, these data suggest that the activity of lurasidone at the 5-HT
7 receptor subtype may mediate some of the antidepressant effects observed in the present study.
Overall, lurasidone as adjunctive therapy with lithium or valproate was well tolerated, with a discontinuation rate due to adverse events that was comparable to placebo (6.0% versus 7.9%). The rate of treatment-emergent hypomania or mania in the lurasidone group was also comparable to placebo (1.1% versus 1.2%), and notably lower than switch rates (approximately 15%) reported in a meta-analysis of mania associated with antidepressant treatment in bipolar disorder (
14). Antidepressants continue to be the most frequently prescribed class of drugs for the treatment of bipolar depression. Their continued use, despite potential safety concerns and lack of consistent evidence for efficacy, underscores the longstanding need to identify treatments for bipolar depression that are effective and well tolerated, including a reduced risk for treatment-emergent manic switching or reduction in cycle length (
14,
15).
In this trial, short-term treatment with lurasidone was associated with minimal effect on weight, lipids, and measures of glycemic control. These findings are consistent with results from previous short- and long-term treatment studies with lurasidone in schizophrenia (
36,
37). Lurasidone has been reported to have notably fewer weight and metabolic effects than other atypical antipsychotic agents (
38). Bipolar disorder is associated with a high incidence of metabolic syndrome (
39) and a significant increase in the risk of cardiovascular disease (
40). The metabolic profile of lurasidone reported in the present study suggests that it may be associated with low cardiometabolic risk in this vulnerable clinical population.
Several study limitations should be noted. Since only patients with bipolar I depression were enrolled, the extent to which these findings can be generalized to patients with bipolar II depression is not clear. Study entry criteria that excluded patients with serious psychiatric or medical comorbidity, and active suicidal ideation or behavior, also may have reduced the generalizability of the results. In this study, the suicide item of the MADRS did not separate from placebo, perhaps in part because of low baseline severity. Finally, further investigation is needed to evaluate the maintenance efficacy of lurasidone and longer-term safety in patients with bipolar disorder.
In conclusion, in patients with bipolar depression, lurasidone added to stable doses of lithium or valproate significantly improved depressive symptoms and associated anxiety symptoms, as well as assessments of quality of life and functioning. Lurasidone appeared to be well tolerated, with minimal effect on weight, lipid parameters, and measures of glycemic control.