Fragile X syndrome, the most common inherited cause of intellectual disability, is the leading monogenic cause of autism spectrum disorder (
1). Fragile X syndrome results from a trinucleotide CGG repeat expansion (locus Xq27.3), leading to hypermethylation of the fragile X mental retardation 1 gene (
FMR1) promoter region and reduced levels of
FMR1 protein (FMRP) (
2). FMRP is involved in regulation of synaptic plasticity and dendritic pruning, which are both critical in neurodevelopment (
3,
4).
Reduced levels of FMRP are associated with cognitive impairment (
5), as well as social deficits that overlap with characteristics of autism spectrum disorder and social anxiety disorder (
6). Despite similarities, specific characteristics of some social deficits are unique to fragile X syndrome. In the case of eye-contact avoidance, individuals with this syndrome are sensitive to gaze initiation and find eye contact aversive, whereas, in general, individuals with autism spectrum disorder are insensitive to social gaze (
7). Eye-gaze aversion in individuals with fragile X syndrome is also associated with changes in skin conductance (
8), cortisol reactivity (
9), and pupillary reactivity (
10), suggesting hyperarousal (
11).
Eye-gaze avoidance in fragile X syndrome may also be linked to abnormalities in the neural circuitry underlying face/gaze processing, as suggested by aberrant morphology in the fusiform gyrus, superior temporal gyrus, and amygdala (
12,
13). Functional MRI (fMRI) studies of gaze processing (
14,
15) indicate abnormal activation in brain regions supporting visual and social processing (the fusiform and superior temporal gyri) (
14), as well as regions underlying emotion processing (the insula and amygdala) (
15,
16). Therefore, eye-gaze avoidance may be linked to emotional responses to eye gaze, such as social anxiety symptoms known to be present behaviorally in fragile X syndrome (
17).
If social anxiety underlies eye-gaze avoidance in fragile X syndrome, then reducing anxiety with behavioral interventions, such as exposure therapy, may prove to be therapeutic. Such treatments have been shown to be effective in reducing symptoms of social anxiety disorder (
18) and in behavioral shaping, a technique showing promise in fragile X syndrome (
19). Habituation to anxiety-provoking stimuli is critical for successful desensitization, and neural system plasticity is essential for habituation (
20). Little is known about neural system habituation in fragile X syndrome. However, deficits in synaptic plasticity have been established (
4). These deficits may be associated with aberrant neural system habituation, and understanding the extent of any habituation impairment will be important for developing behavioral treatments.
Neural system habituation is routinely measured by quantifying changes in the magnitude of fMRI activation in response to repeated stimuli, as demonstrated in typically developing individuals within systems underlying emotion processing (
21). Given the association between FMRP and synaptic plasticity, we hypothesized that individuals with fragile X syndrome would display aberrant neural system habituation. We used an fMRI paradigm with repeated presentations of faces to examine neural system habituation in response to eye gaze in fragile X syndrome. We included faces with direct and averted eye gaze to test a secondary hypothesis that habituation is abnormal in response to direct but not averted eye gaze. We included individuals with fragile X syndrome from both sexes because previous fMRI studies have examined either only female (
14) or only male (
15) subjects, although both studies reported aberrant neural systems underlying gaze processing. We sought to describe effects associated with fragile X syndrome regardless of sex and irrespective of IQ, autism symptoms, and adaptive functioning. Therefore, our comparison group was matched to the fragile X syndrome group on these criteria. As such, differences in habituation could be attributed primarily to fragile X syndrome and not to behavioral symptoms. We also sought to investigate the relationship between neural system habituation and individual differences in the level of FMRP and autism symptoms.
Method
Participants (ages 15–25 years) were 30 individuals with fragile X syndrome and a comparison group of 25 individuals without fragile X syndrome. Fragile X syndrome diagnosis was confirmed through Southern blot DNA analysis (Kimball Genetics, Denver). One female participant and three male participants showed evidence of mosaicism (with both premutation and full mutation); the remaining individuals were diagnosed with full mutation. Blood was drawn from each individual in the fragile X syndrome group to estimate FMRP percentage. Analysis was based on the percentage of peripheral lymphocytes containing FMRP, as assessed by immunostaining techniques (Kimball Genetics, Denver) (
22). Saliva samples were taken from each individual in the comparison group, and polymerase chain reaction analyses were performed to exclude the possibility of fragile X syndrome diagnosis (
23). Participants in the comparison group were diagnosed with idiopathic developmental delay, intellectual disability, or learning disability and were free from any other known genetic condition, premature birth (<34 weeks), low birth weight (<2,000 g), and any serious medical or neurological condition affecting growth and development, including seizure disorder, diabetes, and heart disease. All participants were free from MRI contraindications and met screening criteria for the ability to tolerate fMRI procedures (e.g., the ability to hold still, minimal sensitivity to loud noises, and a cognitive level adequate to complete the behavioral component of the imaging task).
Participants with fragile X syndrome were recruited from across the United States and Canada, and participants in the comparison group were recruited from across Northern California. Both groups were recruited through advertisements, referrals, and word-of-mouth. Nation-wide groups (for fragile X syndrome) and regional centers (for the comparison group) were also used. Participants and/or parents provided written, informed consent and assent to participate in the study. The Stanford University Institutional Review Board approved all protocols.
The groups were matched on sex, intellectual functioning (IQ, assessed using the Wechsler Abbreviated Scale of Intelligence, for ages ≥17 years [
24] or the Wechsler Intelligence Scale for Children, for ages <17 years) [
25]), autism symptoms (assessed using the Autism Diagnostic Observation Schedule [
26]), and adaptive behavior (assessed using the Vineland Adaptive Behavior Scales [
27]). Assessments were administered and scored by qualified personnel based on standard procedures listed in each testing manual. The Autism Diagnostic Observation Schedule was administered and scored by individuals trained by a research certified administrator, and reliability was verified by consensus scoring.
Demographic characteristics and assessment scores for the participants who were included in the fMRI analysis (fragile X syndrome group, N=27; comparison group, N=24) are summarized in
Table 1 (exclusion criteria are presented below). The groups did not differ significantly with regard to sex, IQ, or scores on the Autism Diagnostic Observation Schedule or Vineland Adaptive Behavior Scales.
Participants were scanned on a 3-T General Electric Signa scanner (General Electric Medical Systems, Milwaukee) using one of two custom single-channel quadrature head coils (one head coil was decommissioned during the study). The number of participants scanned with each head coil did not differ between groups (N=51; χ2=1.06, df=1, p>0.10), and head coil type was not related to the within-group blood-oxygen-level-dependent (BOLD) signal.
Whole-brain T
2*-weighted gradient-echo spiral images were acquired with high-order shimming (echo time=30 ms; repetition time=2,000 ms; flip angle=80°; field of view=22 cm; acquisition matrix=64×64; approximate voxel size=4.0×3.4×3.4 mm; 30 axial-oblique slices: 4.0 mm thick, 1.0-mm skip [
28]). Participants viewed color photographs of four college-aged models (male, N=2; female, N=2; Caucasian, N=2; African American, N=1; Asian, N=1) with neutral expressions (see Figure S1 in the
data supplement accompanying the online version of this article). Photographs were selected from previous studies because they elicited differential activation patterns in individuals with fragile X syndrome (
14,
15). Each face was presented 16 times, eight times in each of two gaze orientations (directly toward or averted away from the participant) per run. Two identical consecutive runs lasted 430 seconds each. Habituation was defined as reduced activation in run 2 relative to run 1 (
29). Each face was presented for 4 seconds (two multiples of the repetition time), separated by fixation periods during which participants were instructed to look at a fixation cross presented on a blank screen. Facial stimuli were presented in a fixed pseudo-random order, with fixation durations ranging from 2 to 10 seconds, creating a jittered design with a variable interstimulus interval. Participants were instructed to press button 1 if the person in the picture was looking at them or button 2 if the person was looking away. Responses and reaction times were collected using a button box. High-resolution T
1-weighted anatomical images, collected during the same imaging session, facilitated normalization to standard space (echo time=6 ms; repetition time=35 ms; flip angle=45°; field of view=24 cm; slice thickness=1.5 mm, 124 coronal slices; matrix=256×192; acquired resolution=0.94×1.25×1.5 mm).
All participants practiced lying motionless in an MRI simulator and experienced the sounds and sights of an MRI scanner (
30). Practice continued until they could remain motionless (movement <1 mm, measured by potentiometer) for 10 minutes. Participants also practiced the gaze habituation task with faces not used in the actual fMRI task.
Image preprocessing using statistical parametric mapping software (SPM8, Wellcome Trust Centre for Neuroimaging, United Kingdom) included realignment and correction for motion and global-signal intensity artifacts using the ArtRepair toolbox (
http://cibsr.stanford.edu/tools/methods/artrepair-software.html). Volumes with movement >0.5 mm per repetition time or global-signal intensity fluctuations >1.5% were de-weighted and repaired using interpolation between the nearest nonoutlier scans. Participants whose fMRI series required repair for >20% of volumes were removed from analyses. Images were coregistered to the participant’s anatomical image, normalized to the Montreal Neurological Institute template, and spatially smoothed using a 4-mm full-width at half maximum Gaussian kernel.
First-level statistics included fixed-effects modeling (in SPM8) to identify activation in response to direct- and averted-gaze stimuli separately and overall (direct gaze + averted gaze). Habituation was measured as reduced activation in run 2 relative to run 1 for direct gaze, averted gaze, and overall. Group analyses in FMRIB Software Library (
http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL) included nonparametric permutation testing (10,000 random permutations generated) and iterative cluster threshold finding (threshold-free cluster estimation with family-wise error correction for multiple comparisons, p<0.01) (
31) to minimize type II error. Large eye movements or saccades were estimated during fMRI to provide additional evidence of compliance with the task and attention to the stimuli (see the online
data supplement).
Group comparisons for imaging and behavioral data were covaried by age because of the relatively wide range and skewed distribution of age in the comparison group, which tended to include younger individuals (p=0.05). Accuracy and reaction time were compared using repeated-measures analysis of variance between groups; gaze direction and run were within-subject factors, and age was a covariate. Parallel analysis, including only individuals with >50% accuracy, was performed because judging eye gaze is a known deficit among this study population. Pearson’s or Spearman’s correlation was used to examine relationships between habituation and individual differences in clinical measures. Activation or habituation values for specific regions of interest were extracted using the MarsBaR toolbox (
http://marsbar.sourceforge.net/) and the fslmeants command (
http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL).
Discussion
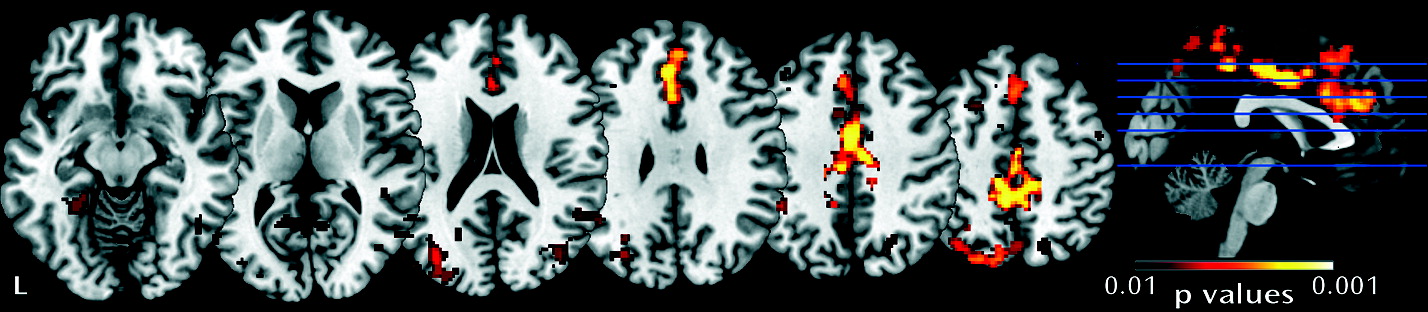
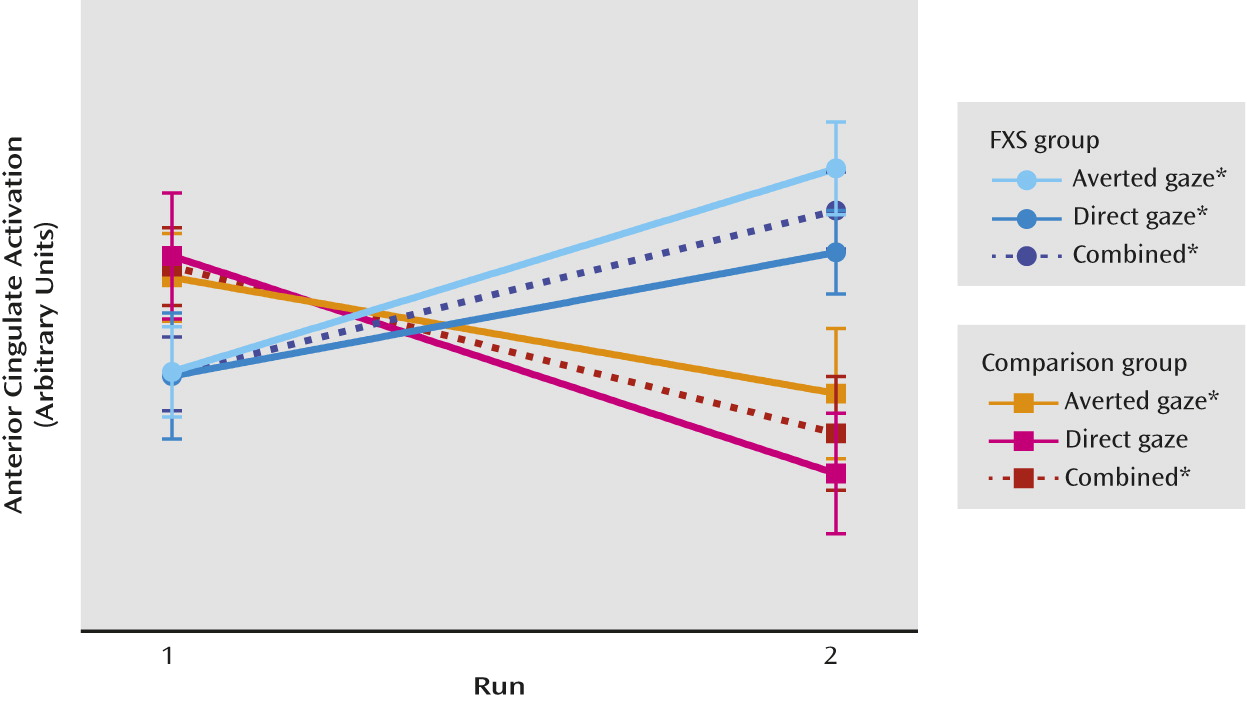
In the present study, we investigated neural system habituation to eye gaze in individuals with fragile X syndrome compared with individuals group-matched for sex, intellectual functioning, autism symptoms, and adaptive behavior. Our primary result reveals less neural habituation in individuals with fragile X syndrome in response to all facial stimuli (direct gaze + averted gaze), and the lack of group difference in activation in run 1 indicates that initial activation differences did not drive this result. There was no evidence for differential habituation to direct gaze compared with averted gaze within or between groups. Less habituation and neural system sensitization was found in the fragile X syndrome group (
Figure 2). This effect was evidenced in widespread cortical regions, including those involved in lower- and higher-level visual processing (the fusiform gyrus and bilateral occipital cortex) and emotion processing (the cingulate), as well as executive functioning regions (frontal). This suggests a deficit in habituation to face/eye gaze that is distributed across multiple levels of neural processing. The regions affected are consistent with findings of abnormal morphology and/or function in fragile X syndrome (
12,
14,
16). Significant correlations in female participants with fragile X syndrome suggest that habituation is related to individual differences in FMRP levels and autism symptoms.
The habituation observed in our comparison group is consistent with that seen in typically developing individuals: decreased activation in response to repeated stimuli (
21,
32) and evidence of neural plasticity reflecting more efficient neural processing (
33). Conversely, the sensitization displayed in the fragile X syndrome group indicates deficient modulation of neural responses to repeated gaze stimuli. Sensitization was seen in regions involved in emotion and social cognition (the cingulate, fusiform, and frontal cortex). One possible interpretation of this result is that neural system sensitization to gaze is related to the inability to modulate arousal (
11). While the link between sensitization and physiological hyperarousal is not directly supported by our data, previous studies suggest support for this hypothesis. Neuroimaging in individuals with fragile X syndrome demonstrates that both social anxiety and gaze durations are related to brain activation in regions supporting social cognition (
34). Atypical eye-gaze behavior has also been linked to aberrant physiology in individuals with fragile X syndrome, including changes in skin conductance (
8), cortisol reactivity (
9), and pupillary reactivity (
10). However, additional factors, such as impaired attention and social cognition, may also contribute to habituation deficits. Furthermore, our design did not allow us to address the specificity of habituation deficits because we did not include nonsocial stimuli. Future investigations of neural system habituation to social and nonsocial stimuli with simultaneous measurement of physiological arousal may help clarify the nature and specificity of habituation deficits.
Further study of habituation in fragile X syndrome could significantly enhance our understanding of associated social avoidance behaviors and provide a useful quantitative measure for evaluating the efficacy of treatments for gaze aversion in fragile X syndrome. Exposure therapy is one treatment technique with the potential to help normalize habituation to eye gaze and reduce gaze aversion. Exposure therapy involves repeated presentations of an aversive stimulus with the goal of decreasing fear or anxiety (
35). Exposure therapy in the form of systematic desensitization has been shown to attenuate symptoms of auditory sensitivity in individuals with autism spectrum disorder (
36). Furthermore, a previous study by our research group indicated that behavioral shaping reduces gaze aversion in fragile X syndrome (
19). These findings suggest that repeated exposure combined with a well-designed behavioral program may facilitate direct gaze. Our present results show increased neural system response to repeated presentations over the course of our relatively brief fMRI experiment, demonstrating plasticity but in the direction of sensitization rather than habituation. Sensitization may be, in part, a result of the social anxiety present in individuals with fragile X syndrome (
17), suggesting that adjunctive pharmacological intervention may prove to be useful in reducing anxiety and physiological arousal, thus enhancing the effectiveness of any behavioral (e.g., desensitization) treatment. In this regard, exposure therapy with graded desensitization to eye gaze may attenuate gaze-aversion behavior, and neural system habituation may be a useful outcome measure.
We attribute deficits in habituation primarily to fragile X syndrome and not to general cognitive ability, autism symptoms, or adaptive behavior level because our comparison group was matched to the fragile X syndrome group on IQ and scores on the Autism Diagnostic Observation Schedule and Vineland Adaptive Behavior Scales. Our finding of differential habituation between groups matched on autism symptoms indicates that the neurobiological mechanisms underlying gaze aversion in fragile X syndrome are different from those underlying similar symptoms in other disorders. These findings underscore the importance of designing disease-specific treatments for fragile X syndrome.
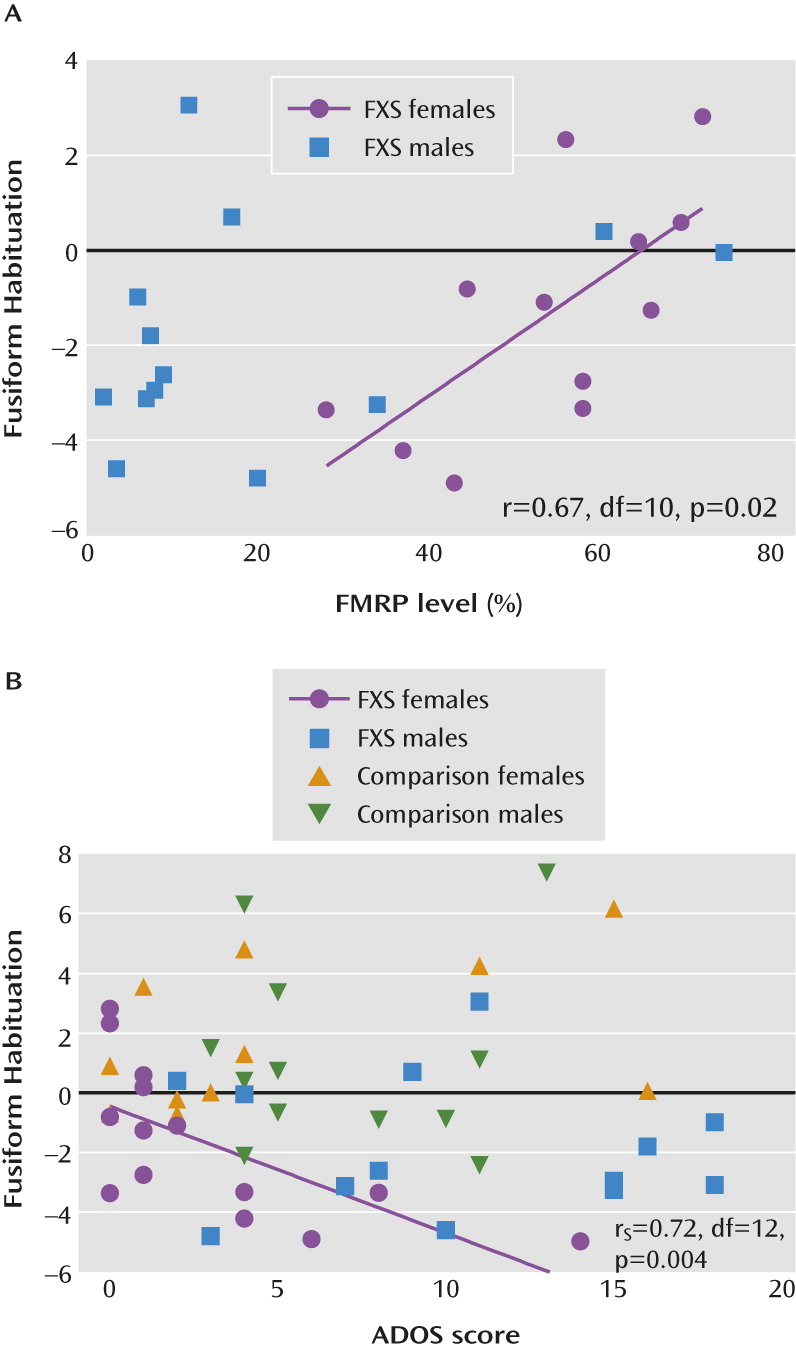
Moreover, we revealed a correlation between the degree of habituation and FMRP levels, suggesting a dose-response relationship with the basic biomolecular component underlying fragile X syndrome. Although correlations with habituation were only significant among female participants with fragile X syndrome, potentially as a result of the skewed distribution of FMRP in males, we note that among the few male participants with FMRP levels >20%, FMRP levels appear to fall along the regression line generated for FMRP levels among female participants (
Figure 3A). Previously, FMRP levels have been correlated with brain morphology in a group of male and female subjects (
13) and with brain function in a study that examined only female subjects (
37). Future studies with larger sample sizes for each sex, and that include male and female subjects with similar FMRP levels, will be important for determining the specificity of relationships between FMRP and neurobiological outcomes. The correlation between habituation deficits and autism symptoms in females with fragile X syndrome supports the theory that habituation deficits underlie gaze aversion and impaired social functioning. Studies directly comparing habituation between individuals with fragile X syndrome and those with similar social deficits, such as autism spectrum disorder and social anxiety, would also be beneficial for determining the specificity of these relationships. Furthermore, examining developmental trajectories of habituation, hyperarousal, and autism symptoms will contribute to our understanding of causal pathways among these related deficits. In the present study, the relationships between habituation and clinical measures (FMRP levels and autism symptoms) were significant only within the fusiform gyrus, a face-processing region important for social functioning and known to have abnormal function (
14,
16) and morphology (
12,
13) in fragile X syndrome.
Interestingly, we did not find significant group differences for habituation in the amygdala, despite its key role in emotion processing. We also did not find a group-by-gaze interaction or any within-group differences in habituation to direct gaze compared with averted gaze in this region. It is possible that our task demands resulted in sustained amygdala activation, and thus habituation or sensitization between runs was not present. This finding is not consistent with those from a previous study by our group, which revealed significant within- and between-group differences in amygdala activation related to gaze direction (
15). However, differences in the experimental design and sampling frame may have contributed to the inconsistent results. Most notably, the present study examined habituation and thus presented a smaller number of unique faces (four compared with 120) for longer durations each (4 seconds compared with 1.75 seconds) over a longer experimental period (approximately 14 minutes compared with approximately 9 minutes). Video clips have been shown to elicit differential responses to gaze direction in individuals with autism (
38) and may have elicited group differences in amygdala habituation and/or a significant effect of gaze direction in individuals with fragile X syndrome.
Our estimate of eye-movement frequency, BOLD signal change in the eye region (see the online
data supplement), indicates that there was no significant group-by-run interaction and no within-group differences in eye movements in run 1 compared with run 2. Thus, a group difference in eye movements did not drive the difference in habituation. We conclude that it is unlikely that group differences in interest or attention were responsible for the differential habituation we observed because such circumstances would be accompanied by changes in eye movements. We observed more eye movements in the fragile X syndrome group than in the comparison group for direct gaze, suggesting atypical responses to direct gaze, perhaps in an effort to avoid direct gaze. More eye movements during direct gaze may have contributed to the lack of difference between habituation to direct gaze compared with averted gaze in the fragile X syndrome group and/or to lack of a group difference within the direct gaze condition for run 1. Future studies using an eye tracker could be used to determine where a participant is fixating and to further delineate face/gaze processing in fragile X syndrome.
Inclusion of individuals receiving medications was a necessary limitation because medication use is high among the populations we studied. Importantly, the fragile X syndrome and comparison groups did not differ with regard to the number of medications used (in general and by class [
Table 1]). Therefore, it is unlikely that the group differences were driven by medication use.
In summary, individuals with fragile X syndrome displayed a deficit in neural habituation and demonstrated sensitization to face/gaze that is distributed across multiple levels of processing. Sensitization in regions involved in emotion and social cognition may be related to an inability to modulate social anxiety, given previous results demonstrating social anxiety and hyperarousal related to gaze processing. Correlation results suggest that habituation is related to individual differences in FMRP levels and autism symptoms assessed outside the scanner. Although the relationships were only significant among female participants, they provide preliminary evidence supporting the relevance of neural system habituation as a biomarker for designing and assessing treatment trials, such as exposure therapy, for gaze aversion in fragile X syndrome.