Cannabis abuse is associated with adverse effects on aspects of adolescent mental health, including cognitive and psychosocial functioning (
1,
2). Also, cannabis smoke has many elements in common with tobacco smoke, and effects in relation to cancer, cardiovascular disease, and pulmonary disease have been documented (
3,
4). Although these medical conditions are in turn associated with excess mortality in the general population (
5), a systematic review by Calabria et al. (
6) did not find sufficient evidence to conclude that the all-cause mortality of cannabis users is increased compared with that of nonusers.
Cannabis use is also associated with nonaffective psychosis (
9). The mortality rate is two to three times higher among persons with such a psychosis, including schizophrenia, than in the general population (
10,
11). With regard to premature deaths, about 40% are explained by suicide or have other unnatural causes, while about 60% have natural causes, such as cardiovascular and pulmonary disease (
10,
12). Although the evidence for an association between cannabis and nonaffective psychosis is robust (
1,
9), it is still unclear whether individuals with nonaffective psychosis with a history of cannabis use have excess mortality compared with those without a history of cannabis use.
Since presence of a psychotic disorder does increase the risk of death, and possibly so also does cannabis use, it is of interest to find out whether there is an interaction effect of the two on risk of death. The interaction of cannabis use and psychotic disorder on mortality rates can be interpreted as either the psychotic disorder affecting the association between cannabis use and mortality, or cannabis use affecting the association between the psychotic disorder and mortality. Both can of course operate simultaneously.
Our aims in this study were to assess 1) the overall risk of death until around age 60 among cannabis users compared with nonusers, as well as the extent to which a psychosis diagnosis during the follow-up affects excess mortality; 2) the overall risk of death among persons with psychotic disorders compared with those without psychotic disorders, as well as the extent to which cannabis use affects excess mortality; and 3) the possible interaction effect of cannabis use and diagnosis of psychotic disorders on mortality.
Method
Subjects
The cohort consisted of 50,373 Swedish men conscripted in 1969 and 1970 for compulsory military training. Over 93% of the men were 18 or 19 years old. Only 2%−3% of men were exempted from conscription, mainly because of a severe mental or physical handicap or a congenital disorder. All of the men completed two nonanonymous questionnaires at the time of conscription. The first covered social background, upbringing conditions, friendships, relationships, attitudes, and adjustment to school and work. The second concerned use of alcohol, tobacco, and other substances. Details of the survey and the methods of the follow-up have been described elsewhere (
16–
18).
All conscripts were assessed by a psychologist after a structured interview. Those presenting with psychiatric symptoms were referred to a psychiatrist, and any psychiatric disorder found was diagnosed according to ICD-8 criteria. Permission to use the conscription database for research purposes and to perform relevant record linkages was granted by the Stockholm Regional Ethical Review Board.
Exposure
Information on cannabis use was obtained from the survey at the time of conscription. In questionnaires, conscripts were asked whether they had ever used drugs, which drugs they had used, the first drug they used, the drug they most commonly used, their frequency of use, and which specific drugs (from a list of possible drugs) they had used.
Information on level of cannabis use was obtained through questions on the number of occasions the subject had used cannabis: never, once, 2–4 times, 5−10 times, 11–50 times, or >50 times. Because of the small numbers of deaths among subjects with a psychotic disorder, we compared outcomes for those having ever used cannabis (thereby putting all subjects who reported any cannabis use into a single category) with persons who had never used cannabis, and also outcomes for those reporting moderate use (once or 2–4, 5–10, or 11–50 times) and those with the highest level of use (>50 times) with those who had never used cannabis.
Follow-Up Data
The cohort of Swedish conscripts was followed from 1969 until 2011. Statistics Sweden maintains records of the unique personal ID numbers of persons in Sweden. We used the personal ID numbers of the Swedish conscripts to link the cohort to data in the registers described below.
The National Cause of Death Register.
This register records all deaths among people registered in Sweden, and it is more than 99% complete. Causes of death are coded according to ICD (ICD-8 for 1965–1986, ICD-9 for 1987–1996, and ICD-10 for 1997–2011) and are divided into the following diagnostic groups: infections: ICD-8 and ICD-9 codes 001–139 and ICD-10 codes A00–B99; cancer: ICD-8 and ICD-9 codes 140–239 and ICD-10 codes C00–D48; endocrine: ICD-8 and ICD-9 codes 240–279 and ICD-10 codes E00–E58; nervous system: ICD-8 and ICD-9 codes 320–389 and ICD-10 codes G00–G99; cardiovascular: ICD-8 and ICD-9 codes 390–429 and ICD-10 codes I20–I52; cerebrovascular: ICD-8 and ICD-9 codes 430–458 and ICD-10 codes I60–I69; respiratory: ICD-8 and ICD-9 codes 460–519 and ICD-10 codes J00–J99; gastrointestinal: ICD-8 and ICD-9 codes 520–579 and ICD-10 codes K00–K99; injuries/accidents: ICD-8 and ICD-9 codes 800–929 and ICD-10 codes V00–V99, W00–W99, and X40–X49; suicide: ICD-8 and ICD-9 codes 950–959 and ICD-10 codes X60–X84; injury undetermined whether accidentally or purposely inflicted: ICD-8 and ICD-9 codes 980–989 and ICD-10 codes Y10–Y34; and other causes: ICD codes not included in the categories above.
The Swedish National Inpatient Register.
The Swedish National Inpatient Register, which covers all inpatient admissions for psychiatric care in Sweden, was used to record the date of first admission to inpatient care for a psychotic episode during the follow-up (1970 until 2011). Diagnoses of psychotic disorders were coded according to the Swedish versions of ICD as follows: schizophrenia: ICD-8 code 295 (excluding 295.50, 295.70); ICD-9 code 295 (excluding 295F, 295H); and ICD-10 code F20; other nonaffective psychoses: ICD-8 code 294.30 for psychosis associated with other physical conditions/drug or poison intoxication; ICD-9 code 292 for drug psychosis; ICD-10 codes F125 and F127 for psychotic disorder due to use of cannabinoids; ICD-8 and ICD-9 code 298 for reactive psychosis; ICD-9 code 293 for transient organic psychotic conditions; ICD-10 code F23 for acute and transient psychotic disorders; ICD-8 code 299.99 for unspecified psychosis; ICD-10 code F28 for other nonorganic psychotic disorders; ICD-10 code F29 for unspecified nonorganic psychosis; ICD-8 and ICD-9 code 297 for paranoia status; and ICD-10 code F22 for persistent delusional disorders.
Subjects with a diagnosis of a psychotic disorder at conscription were excluded from the study.
Migration.
Statistics Sweden maintains a national Register of the Total Population based on information from the Swedish Tax Administration. The register includes data on immigration and emigration since 1966. It has national coverage and is considered to be of high quality.
Outcomes
Overall risk of death among cannabis users compared with nonusers.
Deceased cohort members were identified during the follow-up through the National Cause of Death Register. Overall mortality in the cohort was assessed according to information on the level of cannabis use obtained from the survey at conscription.
Overall risk of death among persons with a diagnosis of a psychotic disorder.
Overall mortality among persons with a diagnosis of a psychotic disorder in the cohort was assessed, through the National Cause of Death Register, according to whether the subjects received a diagnosis of a psychotic disorder in the Inpatient Register during the follow-up. Psychotic disorder was defined as any diagnosis of schizophrenia or other nonaffective psychosis, contingent on the subject seeking and obtaining mental health care. We excluded those who had received a diagnosis of a psychotic disorder at conscription.
Stratified Analyses
First, we assessed the overall risk of death among all subjects with a history of cannabis use compared with those without such a history in the whole cohort, stratified according to whether the subjects received a diagnosis of a psychotic disorder during follow-up (two main categories: subjects without a diagnosis of a psychotic disorder, subjects with a diagnosis of a psychotic disorder). Because of the small numbers of deaths, we compared outcomes for heavy users (more than 50 times) and moderate users (between one and 50 times) with those who had never used cannabis.
Second, we performed a stratified analysis of subjects with a diagnosis of a psychotic disorder, and subjects with the specific diagnosis of schizophrenia, compared with those without such diagnoses, stratified by the three main categories of cannabis users (never users, ever users, heavy users).
Third, since alcohol intake and tobacco smoking are associated with an increased risk of premature death in the general population, we performed stratified analyses that excluded subjects with a history of risky use of alcohol or tobacco smoking at conscription.
Covariates
We selected the covariates listed below on the basis of prior research indicating that they are likely to be associated with our outcome (mortality) and with our exposures (cannabis use, diagnosis of psychosis) (
1–
3,
5–
7,
10–
12,
17,
19–
25). Data on the covariates were obtained from the conscription survey and the psychological assessments at the time of conscription.
Contact with juvenile authorities.
In the conscription questionnaire, subjects were asked whether they had been in contact with juvenile authorities. Responses were categorized as several times, sometimes, or never.
Run away from home.
Questionnaire information was available on whether the subjects had run away from home during childhood, with responses categorized as two or more times, once, or never.
Truancy.
Data on truancy were based on self-reported information from the questionnaire, categorized as once a week, once a month, once per term, or occasionally.
Smoking.
Data on smoking were based on the questionnaire, categorized as >20 cigarettes/day, 11–20 cigarettes/day, 6–10 cigarettes/day, 1–5 cigarettes/day, or nonsmoking.
Solvent abuse.
Data on solvent abuse were obtained from the questionnaire, categorized as >10 times, 2–10 times, once, or never.
Risky use of alcohol.
Information on risky use of alcohol was obtained from responses to questions related to consumption of alcohol: none versus at least one of the following indicators: consumption of at least 250 g 100% alcohol/week; have taken an eye-opener during a hangover; have been apprehended for drunkenness; have reported being drunk often.
Intravenous drug use.
Use of intravenous drugs was based on information from the questionnaires, categorized as several times, once, or never.
Use of other drugs.
Information on the use of other drugs was obtained from the questionnaire, and the following types of drugs were specified: phenmetrazine (a stimulant), amphetamine, LSD, morphine, pentobarbital, and opium. Use of other drugs was categorized as ever used versus never used.
Psychiatric diagnosis at conscription.
Psychiatric diagnoses at conscription were made by a psychiatrist and categorized in this study as any versus none.
Parents divorced.
Data on whether subjects had grown up with divorced parents were obtained from the questionnaire, categorized as yes or no.
Statistical Analysis
Of all 50,373 conscripts, information on cannabis use was missing for 4,998 (9.9%). Thus, 45,375 subjects were included in the final analysis. Table S1 in the data supplement that accompanies the online edition of this article summarizes the baseline characteristics of the initial cohort of 50,373.
Model selection.
Model selection was performed using the R
bestglm package (
https://cran.r-project.org/web/packages/bestglm/index.html). This package uses the lexicographical method suggested by Morgan and Tatar (
26) for general linear models to perform an exhaustive search by first choosing the best model for each number of covariates and then the best of all selected models. The optimality criterion used was the Akaike information criterion. A covariate was included in the model search if it met the criteria for being a confounder (
27). The covariates included in each analysis were selected according to outcomes (overall risk of death among cannabis users compared with nonusers, and overall risk of death among persons with a diagnosis of a psychotic disorder compared with those without) and stratified analyses.
Cox proportional hazards model.
We used Cox proportional hazards modeling to estimate hazard ratios for 1) the overall risk of death among cannabis users compared with nonusers, 2) the overall risk of death among persons with a diagnosis of a psychotic disorder compared with those without, and 3) stratified analyses. Crude and adjusted hazard ratios and 95% confidence intervals were computed. The assumption of proportional hazards was investigated by studying graphs of the log-cumulative hazard functions and the Schoenfeld residuals. The date of first emigration was used as a censoring point. Day of death was used as the primary endpoint.
Interaction.
To test for any interaction effect of psychosis and cannabis use on mortality, we added the interaction between psychosis and cannabis use to the model. We first performed an additive interaction (biological interaction), since it is the most appropriate indicator of interaction in psychiatric epidemiology (
24,
28,
29). We used three measures to estimate additive interaction between cannabis use and diagnosis of a psychotic disorder with regard to mortality: relative excess risk due to interaction, attributable proportion due to interaction, and the synergy index. We calculated 95% confidence intervals for all three measures. A confidence interval including zero in the relative excess risk due to interaction and in the attributable proportion due to interaction indicates no interaction, as does a confidence interval including 1 in the synergy index (
30). We calculated the three measures of interaction with confidence intervals by using a SAS program from Lundberg et al. (
31). Multiplicative interaction between cannabis use and diagnosis of a psychotic disorder with regard to mortality was also tested. The accepted threshold for statistical significance was 0.05.
Causes of death.
In the analysis of causes of death by reported cannabis use, chi-square tests were used to test the differences between never users and moderate users (once to 50 times), and between never users and heavy users (>50 times). Linear hypothesis testing was performed to calculate the p value for trend for a dose-response relationship.
The analyses were performed in SAS, version 9.1, and R, version 3.0.2.
Discussion
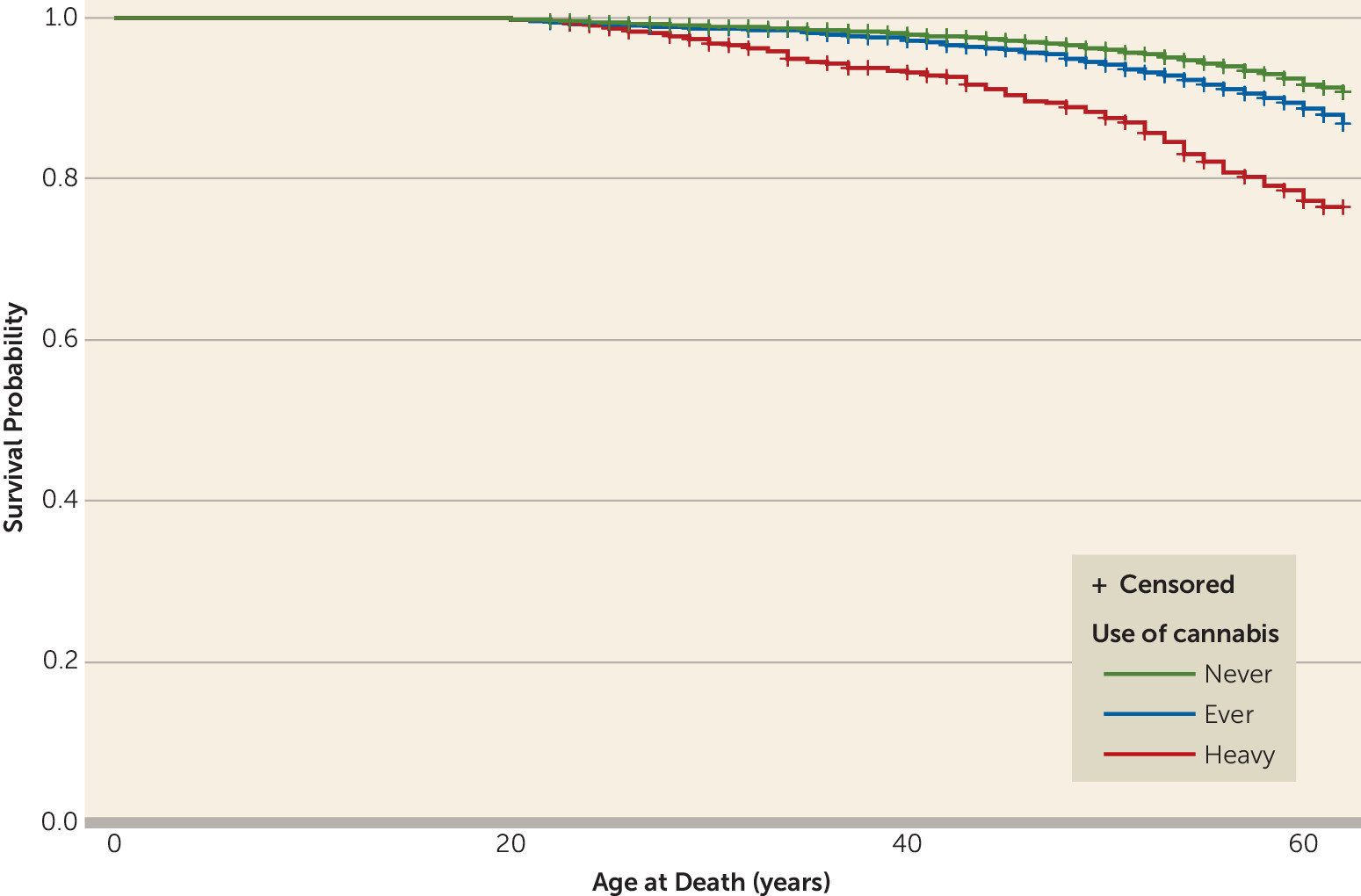
In this long-term follow-up of Swedish male conscripts, we found that those with a baseline history of heavy cannabis use had a significantly higher risk of death (40%) over the 42-year follow-up period than those without a history of cannabis use. The association persisted after controlling for several possible confounders.
Our findings may seem surprising in light of a previous study of this cohort (
7) in which cannabis use was not found to be associated with an increased risk of death. However, in this longer-term follow-up, the cohort members had reached an age where the detrimental somatic effects of cannabis use were more likely to be apparent.
Even though our study shows that subjects with an early history of cannabis use had a significantly higher risk of death even after excluding those with an early history of tobacco smoking and risky use of alcohol, its results should be interpreted with caution. In particular, there is a lack of information about cannabis use, tobacco smoking, and risky use of alcohol for the period after conscription.
It is possible that heavy users of cannabis at baseline continued using cannabis during follow-up, and that this had the strongest effect on mortality at greater ages. The cumulative evidence does suggest that early initiation of cannabis use increases the chances of becoming a daily or nearly daily user (
32,
33). About 10% of persons who ever use cannabis, and one-third to one-half of those who use it daily, will become dependent (
34) and continue using it despite experiencing problems (
32,
34).
Persons at the greatest risk of cannabis dependence have a history of poor academic achievement, deviant behavior in childhood and adolescence, rebelliousness, and poor parental relationships (
1). Although we adjusted for several of these confounders at baseline, it is possible that adverse events in adulthood associated with cannabis use affect the results, which should therefore be interpreted with caution.
We found that the group of subjects with a baseline history of moderate or heavy cannabis use had a significantly higher percentage of deaths due to “injury undetermined whether accidentally or purposely inflicted” than the group without a baseline history of cannabis use. Although the number of persons is small, it is still possible that cannabis use might trigger impulsive acts, ultimately leading to death. Cannabis users have been found to have higher rates of hospital admission for injuries from all causes (
1) and of fatal traffic collisions (
19) compared with nonusers.
However, we did not find an increased risk of suicide among cannabis users, as was the case in a previous study using the same longitudinal population as our own (
22). A review by Serafini et al. (
35) showed that the association between cannabis use and suicidal risk is not robust in the literature and is also inconsistent in relation to completed suicide (
22,
36,
37).
The relation between cannabis use and cancer has been investigated in a number of reviews (
4,
20). Evidence for a link is conflicting, but there is reason to suspect that cannabis use can cause some forms of cancer, including lung cancer. In relation to cardiovascular fatalities related to cannabis use, research findings suggest that cannabis may cause death in individuals with existing vulnerability (
38,
39), but more evidence is needed before any firm conclusions can be drawn.
Although our previous study of this cohort (
16) indicated that schizophrenia patients with a history of cannabis use receive a significantly larger amount of inpatient care, both psychiatric and somatic, we did not find that a history of cannabis use increased the risk of death in subjects with psychotic disorders, and we did not find an interaction effect between cannabis use and diagnosis of psychotic disorders with regard to mortality. We are limited in this respect in that we did not have data regarding the treatment of psychotic disorders or substance abuse in the inpatient register. The introduction of second-generation antipsychotics in the 1990s may have decreased the risk of death among individuals with psychotic disorders who use cannabis. This has been suggested in a review by Wobrock et al. (
40), in which second-generation antipsychotic agents were shown to have greater efficacy than first-generation antipsychotic agents in reducing substance use in patients with schizophrenia.
Medication is not the only factor that can affect the association between cannabis use and risk of death among subjects with diagnoses of psychotic disorders. Other factors, such as psychological and social support and type of care, may also have an impact (
23).
Our study has several limitations, and the results should be interpreted with caution. First, since our information on cannabis use is based solely on a survey at conscription, the study fails to account for differences in lifetime use of cannabis. Nevertheless, performing a long-term longitudinal study does make it possible to conduct analyses of risk of death up to old age in relation to background factors. The Swedish conscript survey provides for the longest follow-up to date of subjects with data on cannabis use. Replication in other long-term cohorts with data on cannabis use would be valuable, although such cohorts, as shown by Calabria et al. (
6) and Degenhardt et al. (
41), are rare.
Second, the causal pathway between cannabis use and risk of death is complicated. Even though cannabis use was assessed at baseline, we do not know the extent to which subjects continued use into adulthood. It is possible that other risk factors after baseline influenced our results, such as risky behavior and use of tobacco, alcohol, and other substances.
Third, the National Cause of Death Register does not record the deaths of persons who emigrate from Sweden. However, we found that subjects with a history of cannabis use had a slightly higher rate of emigration than those without such a history (data not shown), so the effect of unrecorded deaths of emigrants would result in an underestimation of the true effect of cannabis use on mortality in this cohort.
Fourth, identification of diagnoses of psychosis was limited to persons in inpatient care. Jörgensen et al. (
42) found that using data only from inpatient care results in lower estimates of incidence rates compared with using both inpatient and outpatient data. This applies especially since the 1990s, when a higher proportion of patients with psychosis have been treated as outpatients rather than inpatients. However, Jörgensen et al. (
35) also showed that 75% of all persons identified with a nonaffective psychosis do appear in inpatient care when a longer time frame is used or when older cohorts are included. Thus, the facts that most of our subjects were treated before the major period of deinstitutionalization in Sweden and that our study has a long observation period suggest that we are likely to have captured a large majority of patients with psychosis in the cohort.
Cannabis use has also been associated with affective disorders. However, the evidence for causal links between cannabis use and affective disorders is less convincing (
9,
43,
44). In another study using the same longitudinal population as this study, we found no association between baseline cannabis use and subsequent risk of affective disorders after adjusting for confounders (
45). Thus, we decided to focus in this study on nonaffective psychosis.
Fifth, we did not have data on the type of treatment received and possible comorbid substance abuse later in life. Thus, we do not know the extent to which the introduction of second-generation antipsychotics during the 1990s influenced our results on the risk of death among subjects with psychotic disorders. Second-generation antipsychotics have been shown to improve effectiveness in the treatment of schizophrenia and comorbid substance abuse (
40), which might decrease the mortality gap between users and never users of cannabis with psychosis.
Regarding the validity of diagnoses in Sweden’s National Inpatient Register, a number of studies have demonstrated the adequate validity of major psychiatric diagnoses, including schizophrenia, in epidemiology (
46,
47).
In summary, we report on what is to date the longest follow-up of subjects with data on cannabis use and mortality. Study limitations notwithstanding, we found that subjects with a baseline history of heavy use of cannabis have an increased risk of death over the course of follow-up. We did not find that a history of cannabis use affects the excess mortality among subjects with psychotic disorders, and we did not find an interaction effect between cannabis use and diagnosis of psychotic disorders with regard to mortality.