Major depressive disorder is a significant medical condition characterized by high morbidity, recurrence, and suicide rates. Stress, especially psychosocial stress involving psychological-social characteristics, such as interpersonal loss or social rejection, is a strong risk factor for depression (
1). According to the kindling theory (
2), psychosocial stressors not only play an important pathophysiological role in triggering the first depressive episode but also provide a long-term sensitivity to stress that perhaps is a mechanism for subsequent recurrences. Indeed, individuals with depression and childhood traumatic stress experienced dysregulation of hypothalamus-pituitary-adrenal (HPA) axis response to stress such that they formed greater stress sensitivity (
3). Furthermore, with multiple episodes, individuals become more vulnerable because of underlying neurobiological mechanisms, and depressive episodes may be triggered by psychosocial stressors of lesser degrees, even occurring autonomously (
2). Stress sensitivity may serve as a long-lasting trait marker of individuals with depression, even those in remission.
The frontal-limbic dysregulation model of depression (
4) suggests that neocortical, subcortical, and paralimbic structures regulate emotional reactivity and affective behavior. However, neuroimaging studies in which negative emotional paradigms were used with functional MRI (fMRI) have been mixed with respect to changes in the anterior cingulate cortex (
5), whereas hyperactivity in paralimbic structures (
6) and hypoactivity in the prefrontal cortex have been observed relatively consistently in depressed patients (
7). Owing to the prefrontal cortex’s special importance in emotional and cognitive processing, it has been suggested that prefrontal cortex dysfunction may contribute to altered negative affect in symptomatic depression.
HPA-axis function provides another approach for examining neural response to stress, and several studies have examined HPA-axis dysregulation in response to stress in depression. Whereas consistently increased cortisol responses to stress have been observed in remitted individuals (
12,
13), the majority of reports in acute depression have pointed to blunted responses (
14), suggesting a state and trait interaction. Several potential confounds may also account for the observed differences between depressed and remitted subjects: sample demographic characteristics (e.g., older individuals tended to exhibit more blunted cortisol responses [
14]), medication, comorbidity status, stress paradigm differences, and the time of assessments (e.g., greater stress reactivity of depression in the afternoon [
14]). Further investigation is needed before firm conclusions can be drawn.
Regional brain function and HPA-axis responsivity have only been concurrently examined in a few studies (
13,
15). In these studies, cortisol levels did not significantly change in response to an emotional task across groups, perhaps because the stimuli were not sufficiently stressful. A meta-analysis of 208 studies indicated that psychosocial stress tasks, especially those with uncontrollability and social evaluative threat (
16), produced robust stress responses. However, the widely used Trier Social Stress Test and the serial subtraction stress-induction paradigm require speaking or alteration of experimental scenes, which causes head movement and hence is not optimal for functional imaging.
To identify both trait- and state-specific effects of depression on psychosocial neural processing, and to address the aforementioned shortcomings, we conducted an fMRI study in which cortisol and neural responses of individuals with current depression, individuals with fully remitted depression, and healthy subjects to a psychosocial stress task were compared. In the context of the kindling theory, we hypothesized that both similar and distinct brain activation patterns would be observed in cortical and subcortical regions in currently depressed and remitted individuals compared with healthy control subjects, representing state-independent and dependent markers of depression on neural responses to stress, respectively.
Method
Participants
Patients with major depressive disorder diagnoses were recruited from the outpatient department of Second Xiangya Hospital affiliated with Central South University in Changsha, Hunan, China. We used posters and advertisements to recruit age-, gender-, and education-matched healthy subjects from two colleges and a Changsha community.
Psychiatric evaluations were conducted independently by two psychiatrists using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (
17). The 17-item Hamilton Depression Rating Scale (HAM-D) (
18) was used to assess depressive symptoms in patients. Because previous studies have already demonstrated significant brain function changes after antidepressant medication treatments (
19), as well as after multiple depression episodes (
20), only patients meeting the DSM-IV-TR criteria for first major depressive episode were included so that any potential confounding effects from previous medication, depression history, and comorbidities could be excluded. Remitted patients were enrolled if they had at least one episode of depression within the past 10 years but did not meet the DSM-IV-TR criteria for major depression within the 30-day period preceding scanning and had a 17-item HAM-D score ≤7 on the scan day. For all three groups, exclusion criteria were 1) any prior DSM-IV-TR Axis I disorder (except major depressive disorder in the depressed and remitted groups); 2) history of taking antidepressants or undergoing psychotherapy; 3) history of alcohol/substance abuse; and 4) any neurological disorder diagnosis, structural brain abnormalities, or contraindications to MRI.
Ultimately, 38 medication-naive, first-episode depressed patients (19 females), 36 remitted patients (18 females), and 38 healthy subjects (19 females) were recruited. They were all right-handed and of Han ancestry. Each participant was aware of the study’s purpose and signed an informed consent form. Individuals whose scans showed excessive head movement (translation >1.5 mm or rotation >1.5°) were excluded from the analyses.
Stress Task
The Montreal Imaging Stress Task, a well-validated inducer of psychosocial stress (
21), was adapted for use with MRI. The Montreal Imaging Stress Task used a block design with three imaging runs (see the
data supplement accompanying the online version of this article). Each run lasted 7 minutes and consisted of three different conditions: a rest condition (30 seconds) without task requirements, a control condition (90 seconds) in which subjects were asked to answer arithmetic questions without time limit, and a stressful experimental condition (90 seconds) in which subjects were asked to answer arithmetic questions with a time limit and a visible progress bar. Investigators provided scripted negative verbal feedback after each run segment via headphones.
Psychological and Physiological Measures
All participants completed the Beck Depression Inventory-II (BDI), a validated self-report scale of depressive symptoms (
22). The Childhood Trauma Questionnaire (
23) was used to assess traumatic experiences in childhood or adolescence, which might serve as prior psychosocial stressors.
Level of subjective stress was assessed immediately before and after each imaging run via a 0–10 visual analog scale (0=absence of stress; 10=maximal stress). Stress level changes were calculated by subtracting pre-stress from post-stress. Saliva samples were collected with a Salivette (Sarstedt, Nümbrecht, Germany) upon participant arrival (Cort1), after a 30-minute rest (Cort2), upon entering the scanner (Cort3), during anatomical imaging (Cort4), after Montreal Imaging Stress Task runs 1–3 (Cort5–7), and upon leaving the scanner (Cort8). To control for circadian fluctuations, the scans were carried out between 2:00 and 5:00 in the afternoon. Cortisol concentration was detected with a human cortisol ELISA Kit (Bio-Swamp, Shanghai, China). We used cortisol increases from Cort4 (i.e., baseline cortisol level before stress task) to Cort8 (the highest cortisol level after task) as the summary measure of cortisol responses, since this approach is not dependent on the accurate timing of repeated cortisol measurements.
Imaging
Scanning was conducted in a 3.0-T Siemens Magnetom Skyra scanner (Siemens Healthineers, Erlangen, Germany). Blood-oxygen-level-dependent data were acquired with an echo-planar imaging sequence (time to repeat=2 seconds; echo time=30 ms; flip angle=80°; field of view=256×256 mm2; 64×64 matrix; 32 slices; 4-mm slice thickness with 1-mm gap). Statistical Parametric Mapping 8 software (Wellcome Department of Imaging Neuroscience, London) was used for imaging data analysis. Images were realigned, normalized spatially to the standard Montreal Neurological Institute template (resampling into 3×3×3 mm3 voxels), and smoothed with a 6-mm full-width at half-maximum Gaussian kernel. For each participant, imaging data were modeled using a block design. Six motion regressors were also included. We computed t maps of contrasts between experimental versus control conditions for each subject.
To assess stress effects, one-sample t tests were performed in all subjects and each group. One-way analyses of variance (ANOVAs) were applied to detect between-group differences (three levels: depressed, remitted, and control) in the whole brain, and post hoc t tests were applied at a regional level to further investigate the data in regions associated with a significant main effect of group. To control for the effects of residual symptoms of remitted individuals on neural responses, BDI scores were included as a covariate when comparing the remitted group with the control group. Imaging results were corrected via family-wise error rate for multiple comparisons (significance at p<0.05).
Discussion
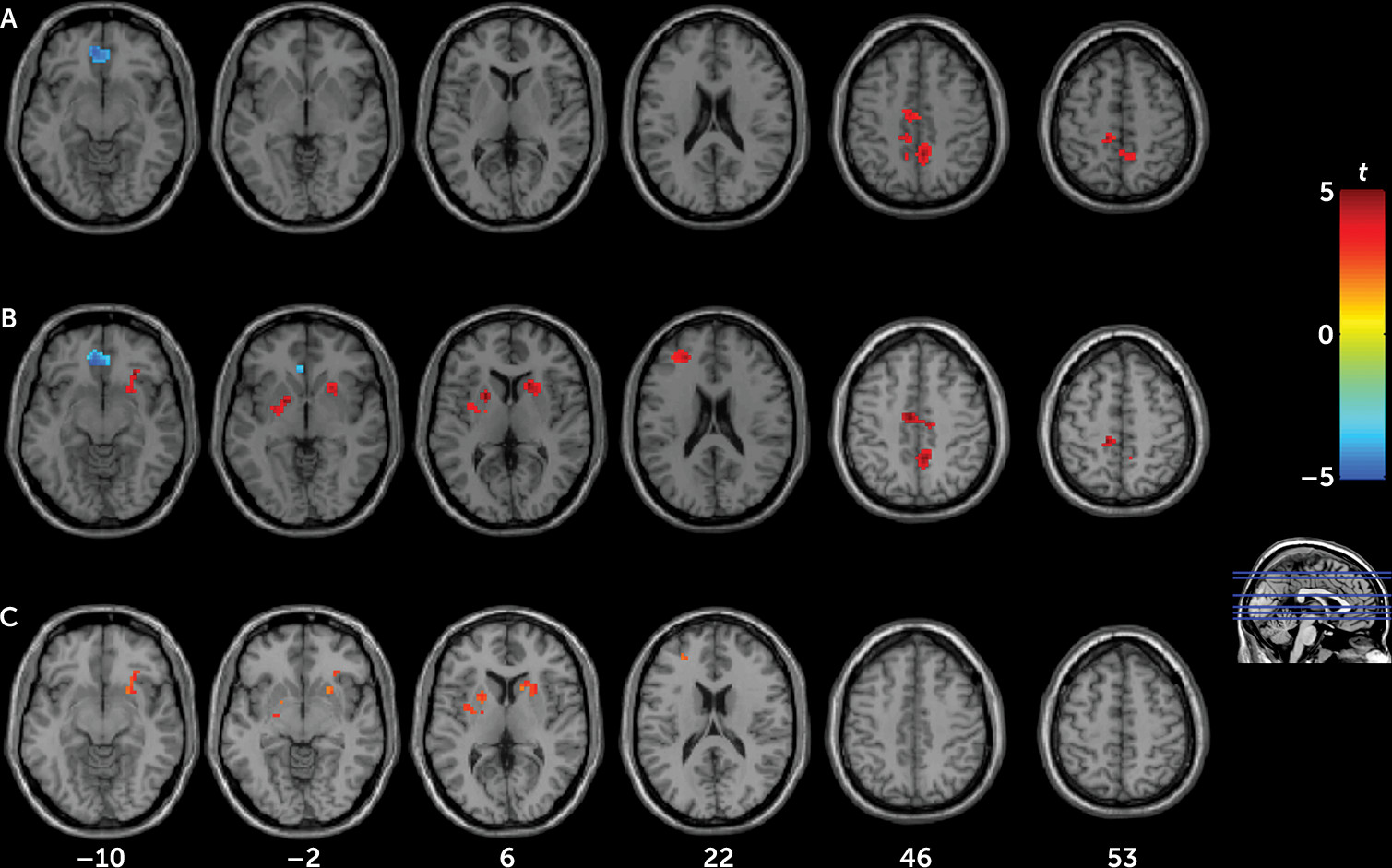
Compared with control subjects, both current and remitted depression patients reacted more strongly to stress and exhibited similar brain activity patterns of hypoactivation of the ventromedial prefrontal cortex and hyperactivation of the precuneus, cingulate, and paracentral lobule, suggesting that these regional brain activation changes may represent a state-independent trait effect of depression that is independent of clinical status. Notably, altered activations in the ventromedial prefrontal cortex and precuneus were also detected after controlling for residual symptoms of remitted patients, indicating that this pattern does not appear to be associated with depressive symptoms per se. In contrast, hyperactivation in the striatum and dorsolateral prefrontal cortex was only observed in remitted patients but not in depressed patients, suggesting that these regional brain activation changes may represent state-dependent neural responses to stress in depression.
Heightened cortisol responsivity to the Montreal Imaging Stress Task was observed in both patient groups, suggesting that HPA-axis hypersensitivity may also be a trait marker of depression, at least in nonmedicated young adults. Notably, although both patient groups had also experienced more childhood traumas than control subjects, the observed brain activity patterns did not change after controlling for Childhood Trauma Questionnaire scores. These results suggest that prior psychosocial stress may primarily act as a “trigger” of depression onset, which is in line with the kindling theory, with greater stress reactivity persisting even in remission.
Our findings of ventromedial prefrontal cortex deactivation with the Montreal Imaging Stress Task in all participants are consistent with findings from a previous study (
25). We also found that the extent of ventromedial prefrontal cortex deactivation correlated with the extent of elevation in cortisol response, linking HPA activity and regional brain function. Similar relationships have been reported previously (
24), supporting a central role of the medial prefrontal cortex in HPA-axis stress-response regulation (
26). The medial prefrontal cortex is known to inhibit the paraventricular nucleus of the hypothalamus, which regulates the pituitary and adrenal release of cortisol. Reduced activation of the medial prefrontal cortex leads to attenuated inhibition of cortisol release (
26). Furthermore, there is a high density of glucocorticoid receptors in the medial prefrontal cortex (
27), and elevated cortisol levels were associated with reduced volume and functioning in this region (
28,
29). Increased cortisol releases and reduced medial prefrontal cortex activations may be a self-reinforcing cycle in depressed individuals, which may limit their capacity for cognitive control and emotional regulation.
Both patient groups showed hyperactivation in the precuneus, middle cingulate cortex, and paracentral lobule, which is consistent with findings from a previous study in depressed patients with stressful life experiences (
30). The precuneus integrates interoceptive and contextual information and hence plays an important role in emotion processing and high-level social cognition (
31). Abnormal precuneus activation may favor self-focused processing and enhance awareness of negative social evaluations. Collectively, both previous (
31) and present findings suggest that precuneus hyperactivity may be a key factor in maladaptive stress-related negative emotionality and social behaviors. Given that cortisol contributes to increased arousal, vigilance, focused attention, and memory formation (
32), hyperactivity in these regions may reflect a facilitatory effect of cortisol on attention focusing, preparation for action, and stress reactivity.
Although significant hyperactivation of the dorsolateral prefrontal cortex and striatum was found in the remitted group compared with the control group, activation in the depressed group was intermediate and not significantly different from either. Based on these results and the negative correlation between depressive severity and the regional activation in the remitted group, which is consistent with prior studies (
33,
34), we speculate that this state-dependent hyperactivation may reflect a compensatory mechanism that may be mechanistically related to remission of depressive symptoms.
The dorsolateral prefrontal cortex subserves executive functions and regulation of emotion (
35). Although findings regarding the dorsolateral prefrontal cortex in acute depression have been inconsistent (
6), hyperactivation of this region has been reported more consistently in nonmedicated remitted depression (
11). Depressed patients also show increased dorsolateral prefrontal cortex activation when taking antidepressants (
36). These findings suggest that remitted individuals may have elevated recruitment of the dorsolateral prefrontal cortex, a mediator of top-down cognitive control, as an adaptation to suppress the negative effects of stress.
Hyperactivation of the striatum in our remitted patient group is consistent with findings from a previous study (
13). Moreover, we found significantly inverse correlations between striatal activation and depressive symptoms or stress-level increases. The striatum is tightly interconnected with the dorsolateral prefrontal cortex; hence it may modulate cognitive processes, such as learning, behavioral execution, and cognitive control (
37). Taken together with previous evidence that increased striatal activation is associated with antidepressant treatments and psychotherapy (
19,
38), hyperactivation of the striatum may also play a protective role in depression remission. However, activation changes in the dorsolateral prefrontal cortex and striatum did not correlate with the duration of depression remission in the remitted group. Future longitudinal studies are needed to determine whether individuals with greater activation in these regions will be able to maintain longer episodes of remission. Moreover, detailed analyses of the functional connectivity between the dorsolateral prefrontal cortex and striatum during stress should be pursued in future studies to explore whether such synergetic mechanisms are involved in remission. Additionally, other cognitive functions, such as working memory and reward sensitivity, that would correlate with the dorsolateral prefrontal cortex and striatum activity should also be measured in future studies, to further characterize the behavioral significance these brain findings have in natural depression remission.
The present study had the advantage of enrolling nonmedicated young adults only, obviating the confounding effects of medication and age. However, several important limitations should be noted. First, we did not control for the menstrual cycle and collect hormonal status data for female participants during the fMRI scan, which may affect their stress responses (
39). The lack of hormonal status data prevents further comparisons between female participants in different phases of the menstrual cycle, and we could not differentiate the potential influences of gonadal and other hormones on neural responses to stress, which need to be studied in future research. Second, cortisol samples were collected between runs, rather than between condition blocks. Cortisol response during the task was determined by calculating the difference between levels in experimental versus control conditions, which should reflect task-induced response well. Third, depression samples without comorbidities in the present study were relatively unique and less representative of community depressed samples. Our findings of trait- and state-like brain activation markers need to be replicated in more general community depressed samples. Fourth, 17-item HAM-D scores were not assessed in healthy control subjects, preventing us from comparing the depressive symptoms in the control subjects and individuals with current or remitted depression. Moreover, our inclusion criterion for duration of remission was 30 days, which was relatively short and might represent only a remitted state, rather than definite recovery. Finally, although our sample size has the power to detect the observed brain activation differences among patients and control subjects, it is still relatively small, and future studies with a much larger sample size are necessary to validate our findings. Future longitudinal follow-ups are also needed to further characterize the persisting and altering neural responses to stress when patients move in and out of depressive episodes.
To our knowledge, this is the first study to investigate neural psychosocial stress processing in current and remitted depression. Compared with control subjects, both patient groups exhibited potentiated cortisol responsivity, decreased activation in the ventromedial prefrontal cortex, and increased activation in the precuneus and middle cingulate cortex in response to psychosocial stress. Dysfunctions of the HPA axis, ventromedial prefrontal cortex, and precuneus during stress may constitute depression trait markers and reflect persistent stress vulnerability. Meanwhile, hyperactivation in the dorsolateral prefrontal cortex and striatum in remitted individuals may be state-specific. We propose that hyperactivation in these regions may augment cognitive regulation during stress, perhaps reflecting compensatory changes. The present study provides new evidence for understanding the neural underpinnings of depression, particularly in response to psychosocial stress.