Much research has focused on the role of anatomical brain abnormalities in ASD (
4–
7). Both larger (
7) and smaller (
8) volumes of striatal structures have been reported in ASD, as well as smaller hippocampal volumes (
9) and, in childhood, larger amygdala volumes (
10). Increased intracranial volume (
11), total gray matter, and cortical thickness have also been reported in ASD (
12), with more specific cortical effects observed mainly in the frontal (
13) and temporal lobes (
14). These structural abnormalities play a crucial role in current theories on the neurobiology of autism. Specifically, altered frontal and striatal volumes and disrupted fronto-striatal connectivity are key components in the executive function deficit theory of ASD (
15–
17). On the other hand, abnormal amygdala volume, specifically in childhood (
18,
19), plays a central role in the social theories of ASD (
10,
20,
21).
However, the neuroimaging literature reflects considerable heterogeneity in the direction and effect size of these morphometric brain differences (
12,
22,
23), with a recent large-scale study by Haar et al. (
12) even indicating overall increased gray/white matter measures but very small local effects of ASD on brain morphometry. This heterogeneity in the literature may be due to various factors. First, variation in case-control differences of brain structures may be due to age differences between study samples. Research has suggested the presence of altered patterns of cortical and subcortical development in ASD, generally with abnormally higher volumes in childhood followed by a more rapid volumetric decline during adolescence and adulthood (
13,
24–
26). Second, factors such as sex, medication use, symptom severity, and presence of comorbidities may also affect case-control differences in brain structures. Third, method-based factors, such as variation in data acquisition, processing, and analysis protocols, may influence the results reported across different studies.
The present study addresses several of these issues. This collaboration was established as part of Enhancing Neuroimaging Genetics Through Meta-Analysis (ENIGMA), the worldwide imaging genetics consortium aimed at unifying analytic methods across a range of neuropsychiatric disorders. We use the ENIGMA processing and analysis pipelines to merge individual subject data from 49 existing ASD case-control cohorts (of which 16 cohorts were collected previously as part of the ABIDE consortium [
6], and 17 as part of ABIDE-II [
30]) to determine whether and which changes in subcortical volumes and cortical thickness and surface area underlie the ASD phenotype across the lifespan. By unifying processing and analysis, we were able to eliminate a large part of the methodological noise between individual studies. Additionally, we were able to investigate directly the effects of sex, IQ, and symptom severity across this extensive sample. Last but not least, studies employing small sample sizes are liable to overestimate effect sizes (
31). With 43 of the 49 included cohorts employing samples of fewer than 100 subjects each, it is important to test whether results of small-scale studies remain robust within this large cohort.
Results
Case-Control Differences in Subcortical and Cortical Partitions
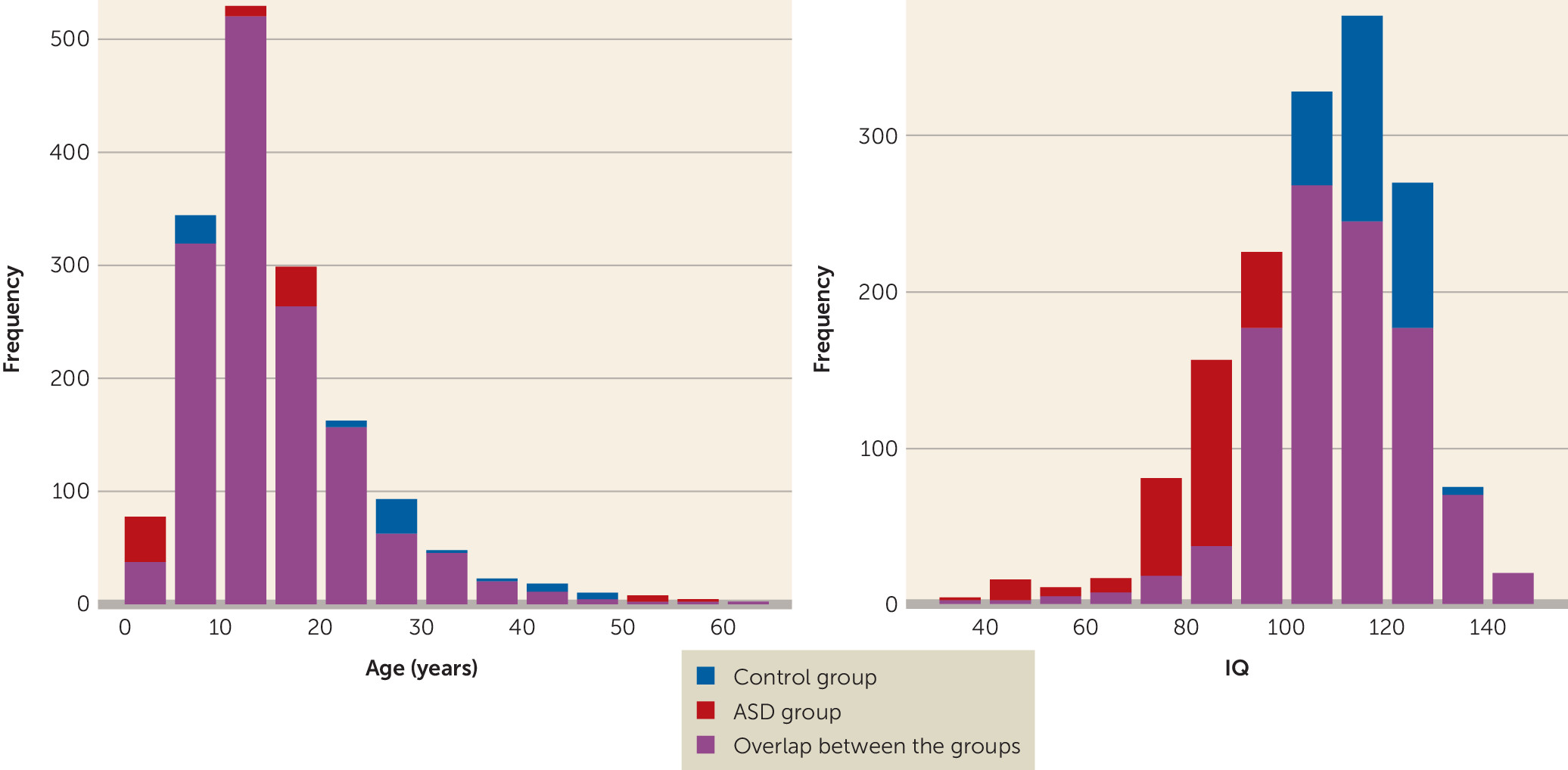
Sex and IQ were not distributed equally between the ASD and healthy control groups. The control group had a larger proportion of females than the ASD group (23.8% compared with 14.25%; p<0.001), as well as a significantly higher mean IQ (111 compared with 103, p<0.001). Both sex and IQ were incorporated as covariates in the main mega-analysis to correct for these differences. Additional sensitivity analyses without correction for IQ as well as analyses with subgroups balanced on sex and IQ are reported in the online data supplement. The effects of medication use and associations with symptom severity were also investigated.
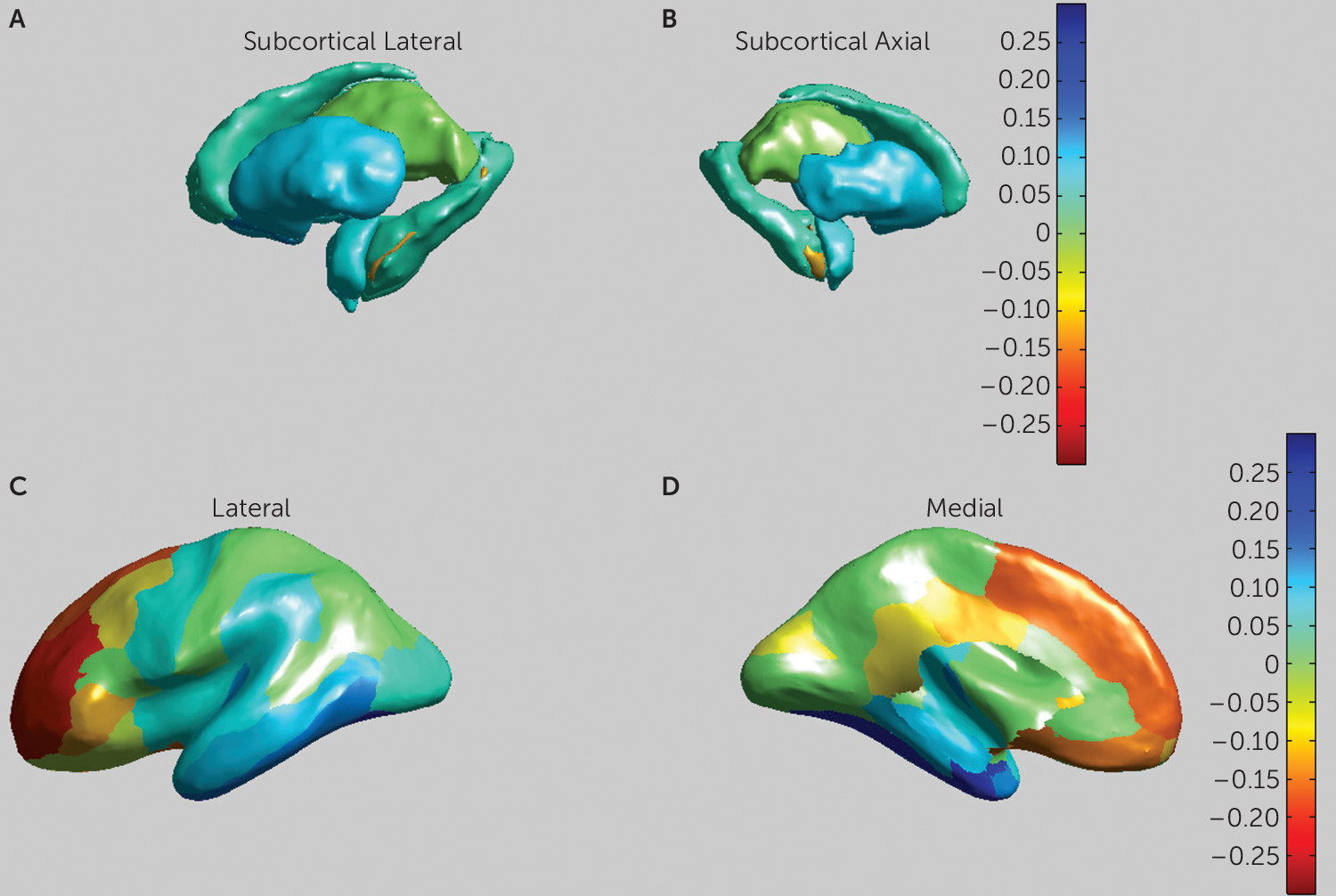
The results of the subcortical mega-analysis are presented in
Table 2 and
Figure 2. Even though the hippocampus is technically a cortical area, we have chosen to add hippocampal volume to the subcortical results, since it is segmented as a subcortical volume in FreeSurfer. ASD was associated with significantly smaller mean volumes of the putamen (p<0.001, d=−0.10), the pallidum (p<0.05, d=−0.08), the amygdala (p<0.05; d=−0.08), and the nucleus accumbens (p<0.001, d=−0.13), as well as a larger mean volume for the lateral ventricles (p<0.001, d=0.11) and a larger mean intracranial volume (p<0.001, d=0.13). Additional sensitivity analyses correcting the subcortical volumes for intracranial gray matter volume instead of total intracranial volume are reported in the
data supplement.
The effects of ASD diagnosis on cortical thickness are presented in
Table 2. We observed a significantly increased overall cortical thickness (p<0.003, d=0.41) in the ASD group, as well as more specifically in nine of the 34 cortical partitions, located in the middle and superior frontal, orbitofrontal, inferior frontal, and posterior cingulate areas. We observed decreased cortical thickness in the ASD group in seven partitions, located in the temporal, entorhinal, and parahippocampal areas (see
Figure 2). We also found increased overall gray matter volume (p<0.052, d=0.23) in the ASD group. No effects of ASD diagnosis on cortical surface area were found.
Post hoc analyses per hemisphere indicated that for the lateral ventricles (left, p<0.004; right, p<0.006), the putamen (p<0.03, p<0.05), and the nucleus accumbens (p<0.008; p<0.02), both hemispheres contribute to the overall effect. For the hippocampus and amygdala, only the right hemisphere showed a significant effect (p<0.05 and p<0.03, respectively). Increased cortical thickness in the ASD group was observed in the frontal cortex for both hemispheres, as well as decreased thickness in the temporal cortex. In the right hemisphere, significantly thicker cortex in the ASD group was furthermore observed in the cingulate cortex, and decreased thickness in the parietal cortex (see Table S8 in the data supplement).
To investigate differential effects between sites, a meta-analysis was performed by treating every site independently and aggregating the results. The results from the case-control meta-analysis confirmed smaller pallidum (p<0.04, d=−0.09), amygdala (p<0.03, d=−0.09), and nucleus accumbens (p<0.01, d=−0.1) volumes in the ASD group, as well as a larger volume for the lateral ventricles (p<0.003, d=0.13) and a larger intracranial volume (p<0.016, d=0.06). The meta-analysis also showed increased cortical thickness in the ASD group in three of 11 frontal partitions and decreased thickness in eight of nine temporal partitions as well as in the supramarginal gyrus (see Table S2 in the data supplement). Overall, the meta-analysis showed smaller effect sizes and higher standard error of effect sizes than the mega-analysis. The I2 test indicates moderate to high heterogeneity across sites for all effect sizes (I2=15.19–64.63). Individual test statistics for the case-control comparison per site are listed in the data supplement (see Table S12).
Age Effects
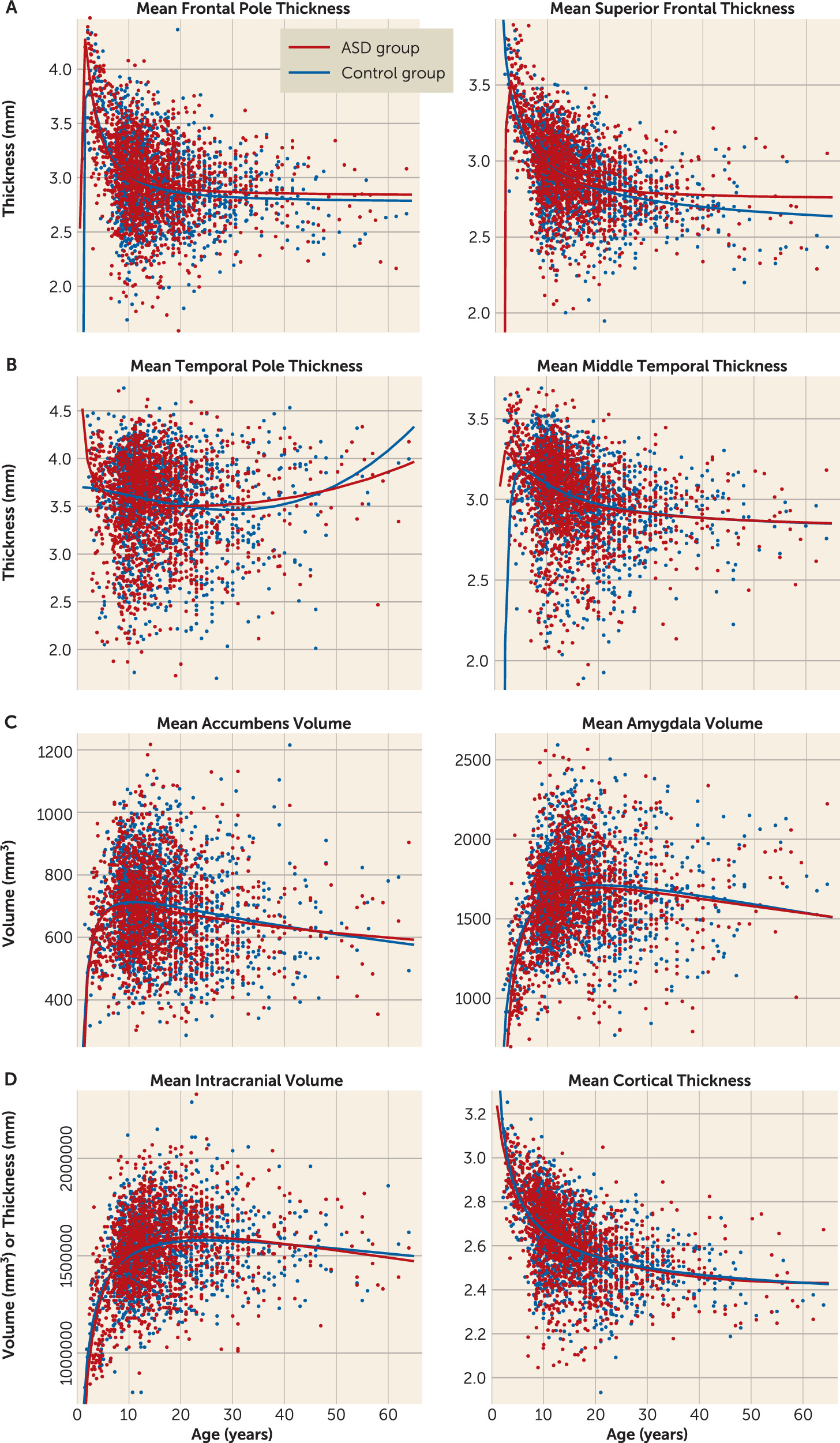
Main linear effects of age were observed for all subcortical volumes (see
Table 2). However, no interaction effects between diagnosis and age were found. We calculated fractional polynomial fits for the age effect for all the above-mentioned regions of interest, estimating the polynomial fit for these volumes (
Figure 3; see also Table S11 in the
data supplement). The fractional polynomial approach indicates that the optimal model for the age effect in all subcortical volumes contains the powers of 0.5 and 2 (see
Figure 2). These results indicate that the developmental curves of the ASD and healthy control groups follow similar trajectories over time, which confirms the observed lack of detectable age-by-diagnosis interactions.
Main linear effects of age on cortical thickness were observed in 30 of 34 partitions, all of them showing a negative relationship between age and thickness (see
Table 1). The four partitions that did not show a significant effect all overlapped with the partitions that showed a negative relationship between thickness and ASD diagnosis (temporal, entorhinal, and parahippocampal areas). A quadratic effect of age was observed in the insula. Age-by-diagnosis interactions were observed in 24 of the partitions that also showed a linear age effect. Fractional polynomial plots were calculated for these partitions as well, showing complex developmental curves, including both quadratic and cubic effects across the partitions (see
Figure 3). These visualizations indicate that individuals with ASD show a peak in cortical thickness differences around adolescence, with both the greater thickness in the frontal cortex and the lesser thickness in the temporal cortex peaking around this age.
Sex and IQ Effects
The mega-analysis shows significant effects of sex on intracranial volume and volumes of the lateral ventricles, thalamus, caudate, putamen, amygdala, and nucleus accumbens, indicating larger volumes in males than females. No interactions between diagnosis and sex were observed. Effects of IQ on brain volumes were observed for volumes of the putamen, hippocampus, amygdala, and nucleus accumbens, with larger volumes associated with higher IQ (see
Table 2). The three-way interaction of diagnosis, age, and sex rendered no significant results.
Effects of sex on cortical thickness were observed in transverse temporal, caudal-middle-frontal, and superior frontal partitions, all of them indicating a thicker cortex in males. Participants with a higher IQ showed greater cortical thickness in precentral and rostral anterior cingulate partitions and lower thickness in medial orbitofrontal and caudal anterior cingulate partitions.
Medication, Comorbidity, and Symptom Severity Effects
Within the ASD group, further mega-analyses were performed to test for any effects of medication use, comorbidities, and ASD symptom severity on subcortical volumes (see the online data supplement). Neither medication use nor the presence of comorbidities significantly influenced subcortical volumes within this sample. Cortical thickness was associated with medication use only in the inferior-temporal partition (d=−0.47, p<0.002), but not with comorbidity. ASD symptom severity analyses showed that higher ADOS scores were associated with larger intracranial and lateral ventricle volumes and lower volumes in the putamen, nucleus accumbens, thalamus, amygdala, and hippocampus (see Table S9 in the data supplement). Greater thickness in the frontal areas and lower thickness in the temporal areas were associated with higher ADOS scores. Interestingly, higher ADOS scores were additionally associated with increased thickness in cingulate, parietal, and occipital regions, while lower thickness in the insula was associated with higher ADOS scores.
Discussion
We investigated subcortical brain volumes, cortical thickness, and surface area in the largest sample to date of patients with ASD and typically developing healthy control subjects across a wide age range. ASD was found to be associated with significantly smaller volumes for the putamen, pallidum, and nucleus accumbens and larger volumes of the lateral ventricles. The ASD group also showed generally larger intracranial volume, total gray matter, and total cortical thickness, but no differences in surface area. Our analyses indicate a split in the direction of cortical thickness effects between the frontal and temporal cortices, where the ASD group showed increased cortical thickness in the frontal cortex but decreased thickness in the temporal cortex. The effect sizes of these cortical and subcortical group differences ranged from −0.21 to 0.20, indicating small to moderate effects and significant overlap in the distribution of brain morphometry measures between the ASD and control groups, in line with effect sizes reported by the ENIGMA attention deficit hyperactivity disorder (ADHD), schizophrenia, and bipolar disorder working groups (
34–
36).
Increased cortical thickness in general, as well as increased lateral ventricle volumes, are in line with results found in the meta-analysis by Haar et al. (
12), although those authors did not find any alterations in basal ganglia volumes or frontal cortical thickness. Decreased temporal cortical thickness and increased frontal thickness, however, were observed in another recent large-scale meta-analysis on brain morphometry in ASD (
37). Our sensitivity analyses, based on the permutation testing used in the Haar et al. (
12) study, indicate that the discrepancies with the present results can be attributed mainly to differences in sample size, as we replicate previous results using the permutation method on our larger cohort sample. This comparison further indicates the necessity of large-scale cohort studies and stresses the caution needed when interpreting effects with these limited effect sizes.
The involvement of the pallidum, putamen, and nucleus accumbens indicates an important role for the socio-motivational and cognitive and motor systems of the striatum in the neurobiology of ASD (
38). Taken together with the increased cortical thickness in the frontal cortical regions, which are involved mainly in cognitive control, these findings are in line with previous studies relating aberrant frontal-striatal connectivity to the repetitive behavior and executive functioning deficits observed in ASD (
6,
14,
17,
39). Nucleus accumbens deficits have additionally been suggested to support the theory of social reward–based differences underlying part of the behavioral phenotype in ASD (
15). In contrast to some earlier findings (
19), but in line with others (
10), we also found a slightly smaller amygdala volume in ASD. Further research is needed to investigate whether changes in nucleus accumbens and amygdala volumes are related to social and reward processes in ASD. Decreased thickness in the temporal cortex in ASD may be further related to both social (
40) and language deficits in the disorder (
41). Our post hoc analyses on ASD severity further indicate that cortical and subcortical morphometry is related to ASD severity, following the same direction as the group effect, indicating that these alterations indeed serve a functional role in the ASD etiology.
The age distribution in the present sample ranged from 2 years to 64 years, providing an unprecedented cross-sectional view of the development of brain morphometry in ASD and healthy control subjects over the lifespan. Our subcortical results indicate complex—linear and quadratic—age effects in the thalamus, hippocampus, amygdala, nucleus accumbens, and lateral ventricles. The fractional polynomial fits for these volumes indicate that across both diagnostic groups, the subcortical volumes follow a quadratic growth model with a distinct peak around puberty, which is in line with previous analyses of the development of these regions (
42). As opposed to some previous studies and reviews (e.g.,
26), we found no evidence for age-by-diagnosis interaction effects in any of the investigated subcortical volumes (
43). Cortical thickness, on the other hand, showed large-scale effects of both age and age-by-diagnosis interaction. We observed a general decline in cortical thickness over age, in line with previous studies (
13,
32). However, as compared with the control subjects, the ASD patients in general showed the strongest group differences during childhood and adolescence, with normalized or even reversed thickness results in adulthood. Interestingly, this was observed both for the increased thickness in the frontal areas and the decreased thickness in the temporal lobe in ASD. These results indicate a complex maturation pattern for the subcortical, frontal, and temporal structures in ASD, peaking around adolescence. Specifically, the balance of frontal, temporal, and striatal maturation may prove a valuable marker for the development of ASD symptoms and treatment response, although further longitudinal studies are needed to verify the predictive validity of these morphometric measures.
Our findings also replicate previously reported main effects of sex (
44) and IQ (
45) on brain volumes, with generally larger subcortical volumes and increased thickness found in males and in participants with a higher IQ. The mega-analytic approach can statistically correct for differences in sex and IQ between participating sites, removing some of the outcome variance associated with different distributions of these factors between sites. We observed that males have, on average, larger basal ganglia volumes and increased cortical thickness, but the patient groups had a larger proportion of males, indicating that sex could not have confounded the ASD effect. Although we found no evidence of a sex-by-diagnosis interaction, the increased volumes and thickness in both males and females with ASD could be taken as evidence for the “extreme male brain” hypothesis (
46). Although our study was not optimally designed to validate this hypothesis, other recent large-scale studies have found considerable evidence for gender effects in brain morphometry in ASD (
47). We aim to replicate the analysis by Ecker et al. (
47) in a future report on the ENIGMA ASD cohort. Neither medication use nor comorbidity had any large-scale influence on brain morphometry in ASD. Within the ASD sample, overall effects of symptom severity were largely consistent with the direction of the between-group effects. This supports the inference that the observed differences in morphometry are indeed related to the phenotypic expression of ASD in this cohort.
Since individual participant data were available for almost the entire sample, we could compare meta- and mega-analytic approaches in this data set. The main difference between these approaches is that in a meta-analysis, within- and between-group variance is estimated for each separate site, whereas for the mega-analysis, variance is estimated within group. Our results indicate that the meta-analysis is generally less sensitive to group differences, with smaller effect sizes and higher standard errors. The meta-analysis also allowed us to investigate the effects of ASD on brain morphometry per site (as presented in the online data supplement). This analysis indicates significant heterogeneity in the direction and size of the effects between participating sites. The heterogeneity of effect sizes as expressed with the I2 measure was moderate to high for all volume differences found.
This heterogeneity is very important to take into account when interpreting single-sample studies. Even though all the data in the present study were processed using the same analysis and quality-control pipeline and were corrected for effects of age, sex, and IQ identically across sites, we still found significant differences in the estimated main effects between sites. Some of these differences may be due to random sampling differences of the ASD population, in particular within the smaller samples. However, this is less likely to be the case in larger samples employing hundreds of subjects. Alternatively, the within-group heterogeneity may be indicative of different biological mechanisms or subtypes underlying ASD and may therefore be informative for further classification studies. In any case, the existence of this heterogeneity underlines the importance of large-scale studies such as ENIGMA in developing reliable benchmarks for the different major psychiatric disorders. The establishment of these benchmarks allows us to more accurately tackle the heterogeneity due to measurement differences and biological differences in neurodevelopmental studies. Planned multivariate factor analyses and subtyping analyses may additionally provide further insight into different biological mechanisms in ASD.
One of the main goals of the ENIGMA consortium is to unify analytical methods not only across samples, but also across different disorders. The recent report of the ENIGMA ADHD working group (
36) is based on the same analysis pipeline and mega-analysis as the present study, using a similarly sized sample of individuals with ADHD and control subjects. The ENIGMA ASD and ADHD results suggest similar decreases in volumes in the putamen, amygdala, and nucleus accumbens, whereas differences are found in pallidum volume in ASD but not ADHD. Additionally, age analyses of subcortical volumes using fractional polynomials suggest different patterns of neural development in ASD and ADHD. Whereas patients with ASD show volume growth curves similar to those of control subjects, patients with ADHD show a significant diagnosis-by-age interaction, with different developmental models most clearly seen in volumes of the nucleus accumbens and putamen for ADHD patients (
36). These partly overlapping striatal volume differences offer a fascinating starting point for further investigation of the shared and unique neurobiological underpinnings of ADHD and ASD, as direct comparisons between these two cohorts have not yet been completed at the time of writing.
Some limitations should be considered when interpreting these findings. Our various participating sites used different scanners and acquisition protocols, and although we controlled for the effects of scan site in our models, we cannot fully exclude potential influence from these measurement protocols on the data. We were also unable to obtain longitudinal data for the out samples and therefore could not conduct a within-subject analysis of brain development in ASD. Future large-scale efforts should, in our opinion, be aimed at also standardizing acquisition protocols and long-term follow-ups.
In summary, this study showed the abnormal development of cortical thickness and subcortical volumes in ASD in the largest sample to date, as obtained by the ENIGMA ASD working group. In ASD patients compared with healthy control subjects, we observed smaller volumes for the putamen, amygdala, nucleus accumbens, and pallidum, increased frontal cortical thickness, and decreased temporal cortical thickness. Our age analyses show that subcortical differences in ASD remain relatively stable over the lifespan, while cortical alterations in ASD show a peak in childhood and early adolescence and taper off over adulthood. Future functional activation and resting-state connectivity studies will want to take into account these differences in maturation and focus on unraveling how the balance between frontal, temporal, and subcortical alterations influences the expression of the ASD phenotype across the lifespan. No differences in the development of brain morphometry were observed between males and females with ASD.