Prolonged Postconcussive Symptoms
What is the usual prognosis of an acute postconcussive syndrome?
Epidemiology of Prolonged Postconcussive Symptoms
Definitions
Assessment
1. What were the characteristics, context, and mechanism of the mTBI?
2. Is there evidence of traumatic brain damage on neuroimaging?
3. How many previous mTBIs has the patient sustained, when did they occur, and how severe were they?
4. What are the severity and trajectory of prolonged postconcussive symptoms, and what are the demographic, traumatic, and behavioral risk factors for them?
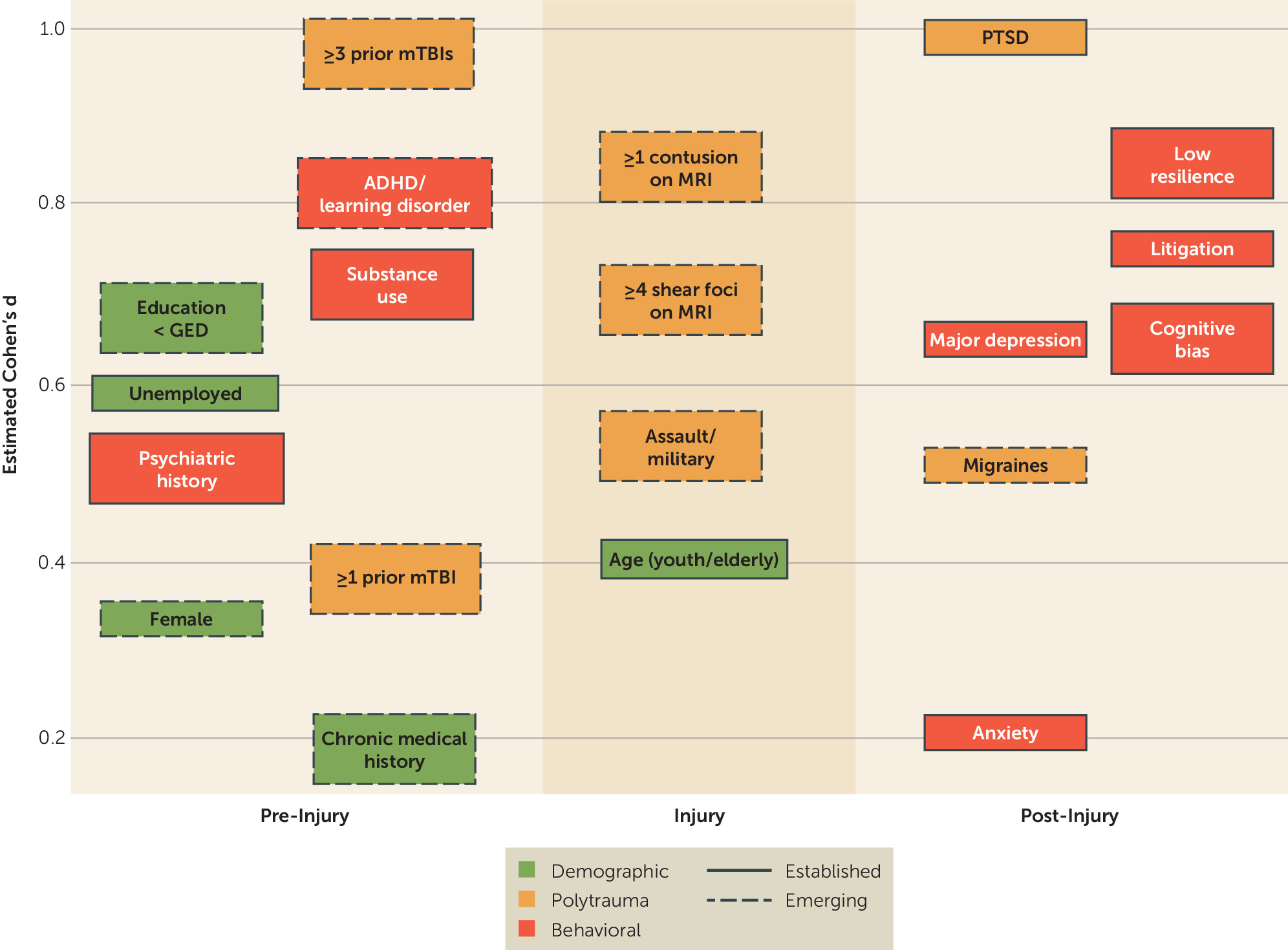
5. Are there other conditions masquerading as prolonged postconcussive symptoms?
Differential Diagnosis
Treatments
Medications
Psychosocial Interventions
Metabolic Treatments
Physical Therapies
Neurostimulation
Conclusions
D. Improvement with combined therapies.
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

