Neurobiological and behavioral heterogeneity is a persistent obstacle to discovery in psychiatric disorders (
1). The traditional case-control study design assumes that a biological marker may differ enough between groups for diagnostic differentiation. This assumption is under debate, because neurological and behavioral heterogeneity appears to be the rule rather than the exception (
2–
4). Such heterogeneity is present not only across psychiatric disorders but also among non-ill, healthy control individuals (
3–
6). For example, variability across individuals is present in functional MRI (fMRI) tasks of memory (
4), and differences have been related to cognitive strategies (
6–
8).
Variability among groups of individuals has recently received attention within the neuroimaging literature, spurred by findings of patterns of atypical activation within a subset of participants (
5); findings of differing patterns of activity associated with distinct neural strategies (
4,
6); and findings that better or poorer performers, identified a priori, may not engage the same neural circuitry (
7,
8). Such studies, however, typically involve small sample sizes and rely on group analyses. With adequate sample sizes and a combination of clinical and biological data acquisition, heterogeneity of neural activity and individual variability may serve as an opportunity for biological discovery (
2,
9–
11). In particular, understanding different patterns of activation among individuals may be an important first step toward using neuroimaging endpoints for prognostic and target engagement studies.
The Research Domain Criteria (RDoC) approach proposed by the National Institute of Mental Health (NIMH) conceptualizes healthy control and patient populations along a continuum, with considerable overlap hypothesized in brain structure, brain function, and cognitive performance (
12). The RDoC initiative has proposed five domains for study; the domain of social processes is composed of four social process constructs (i.e., affiliation and attachment, perception and understanding of self, perception and understanding of others, and social communication), which tend to be impaired in people with schizophrenia spectrum disorders (
13). These impairments in social cognitive processes tend to be present early in the disease course (
14) and are related to, and potentially mediate, the relationship between neurocognition and functional outcome (
15).
The NIMH-funded Social Processes Initiative in the Neurobiology of the Schizophrenia(s) (SPINS) is a multicenter RDoC study (the Centre for Addiction and Mental Health in Toronto, Zucker Hillside Hospital in New York, and the Maryland Psychiatric Research Center in Baltimore) that aims to use large sample sizes, multimodal neuroimaging, and behavioral assessments to identify impairments in neural circuit structure and function that predict impairment in lower-level and higher-level social cognitive processes (
16) across the continuum of people with and without schizophrenia spectrum disorders. We hypothesized that altered structural connectivity in the right frontoparietal (simulation) circuit would be associated with impaired lower-level social cognitive processes, whereas altered connectivity in the cortical midline and temporoparietal (mentalizing) circuit would be associated with impaired higher-level social cognitive processes. Delineation of the key neural circuits underlying these social cognitive processes may identify treatment-relevant subgroups, which may lead to new studies to test targeted brain stimulation, and potential response, in relation to neural strategy.
In this study, individuals recruited as part of SPINS all completed the same in-scanner imitate/observe task (
16–
18), a task that activates the simulation circuit and that also may be associated with mentalizing circuit activity (
17). Additionally, participants completed a battery of social cognitive and neurocognitive tests outside the scanner. We used a hierarchical clustering approach to examine whether fMRI data collected during the performance of the task identified unique subgroups and whether these subgroups differed in performance on the social cognitive and neurocognitive tests. We hypothesized that differences in patterns of social cognitive circuit (both simulating and mentalizing) activation across the identified subgroups would be differentially associated with social cognitive and neurocognitive test performance. We then replicated the analyses in an independent replication sample collected only at the Centre for Addiction and Mental Health.
Methods
Participants
The discovery study sample included participants (ages 18 to 55) who were recruited from the three SPINS sites. Recruitment was initiated in December 2014; recruitment from the Zucker Hillside Hospital and Maryland Psychiatric Research Center sites was halted in April 2016 to align with a scheduled shutdown for scanner upgrades, whereas participants from the CAMH site were included if scanned by October 2016. Participants with schizophrenia spectrum disorders met DSM-5 diagnostic criteria for schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, or other specified schizophrenia spectrum and other psychotic disorder; had been assessed with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-IV-TR) (
18); and had no change in antipsychotic medication or decrement in functioning or support level in the 30 days prior to enrollment. Control participants had no current or past axis I psychiatric disorder, except for adjustment disorder, phobic disorder, past major depressive disorder (more than 2 years earlier and currently unmedicated), and no first-degree relative with a history of a psychotic mental disorder. Additional exclusion criteria included a history of head trauma resulting in unconsciousness, a substance use disorder (confirmed by urine toxicology screening), intellectual disability, debilitating or unstable medical illness, and other neurological disease. The independent replication sample consisted of 108 participants, including 32 individuals diagnosed with a DSM-IV-TR-defined schizophrenia spectrum disorder, 37 diagnosed as having DSM-IV-TR-defined euthymic bipolar disorder, and 39 healthy control participants (exclusions as above), assessed via the SCID-IV-TR, all of whom were recruited and underwent scanning at the Centre for Addiction and Mental Health. The diagnostic status of all participants in both samples was confirmed via the SCID-IV-TR, although DSM-5 diagnostic criteria were applied to the SPINS sample. (See Appendix S1 in the
online supplement for more detailed inclusion and exclusion requirements for all study protocols.) All protocols were approved by the respective site research ethics boards and/or institutional review boards, and all research was conducted in accordance with the Declaration of Helsinki. Participants signed an institutionally approved informed consent form before undergoing any research procedures.
In the SPINS discovery sample, the Brief Psychiatric Rating Scale (BPRS) (
19) and the Scale for the Assessment of Negative Symptoms (SANS) (
20) were administered only to the participants with schizophrenia spectrum disorders. The BPRS total score was used to assess global psychopathology. The SANS items were used to assess the diminished expression and diminished motivation constructs (
21). In the replication sample, the Positive and Negative Syndrome Scale (PANSS) (
22) was used to assess global psychopathology in the individuals with schizophrenia spectrum disorders. All individuals underwent a urine drug screen to confirm absence of current substance use, and none had met DSM-IV-TR criteria for substance dependence or abuse within the past 6 months.
MRI Scanning
MRI scanning was conducted on a General Electric Discovery 3-T scanner at the Centre for Addiction and Mental Health, on a General Electric Signa 3-T scanner at Zucker Hillside Hospital, and on a Siemens Tim Trio 3-T scanner at the Maryland Psychiatric Research Center. The imitate/observe task was part of a longer multimodal MRI protocol to which each participant consented. Each block of the task (imitate or observe) used an echo-planar imaging scan (110 repetition times of 3 seconds, echo time 30 ms, voxel size 3 mm isotropic, 50 slices, 64×64 matrix with field of view=192 mm, flip angle=77°). A fast-gradient echo anatomical T1 scan was collected for use in the preprocessing pipeline (repetition time, 2300 ms, 0.9 mm isotropic, no gap, interleaved ascending).
Imitate/Observe Task
Participants performed the imitate/observe task during scanning as previously described (
23–
25). Participants were shown, in a randomized order, full-color images of 16 racially diverse individuals (eight of them men) expressing five different facial expressions from the MacBrain Face Stimulus Set (
https://www.macbrain.org/resources.htm) (i.e., fearful, sad, happy, angry, or neutral; 16 of each expression for a total of 80 per block) or fixation crosses (16 per block) for 2 seconds, with a jittered interstimulus interval (500 ms to 1500 ms). During two counterbalanced scanning sessions, participants were instructed either to imitate the facial expression (the imitate scan) or to observe the facial expressions (the observe scan). A live video feed during the scan was used to confirm whether or not participants were performing the task.
Social Cognitive and Neurocognitive Assessments
The social cognitive battery was composed of the following tests: the Penn Emotion Recognition Task (
26), which assesses emotional face recognition, measured via reaction time; the Reading the Mind in the Eyes Test (
27), which assesses the ability to judge the complex mental states of others based on a black-and-white image of their eyes; the Relationships Across Domains measure (
28), which assesses the ability to generalize limited information to other aspects of social life; and the Awareness of Social Inference Test, Revised (
29), the three subtests of which examine emotional recognition, theory of mind, and ability to detect deception and sarcasm. The social cognitive battery is further detailed in Appendix S2 in the
online supplement. The MATRICS Consensus Cognitive Battery was used to assess neurocognition (
30) across six neurocognitive domains (speed of processing, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving).
MRI Task Standardization and Quality Control
The imitate/observe task procedures and scan parameters were closely harmonized across sites. Prior to study initiation and annually thereafter, videoconference training was conducted with study staff for standard operating procedures related to MRI scans and participant training. Prior to entering the MRI, participants completed a training and practice session, lying supine while performing the imitate/observe task on 10 faces not included in the study. Practice was repeated until participants imitated the faces without head motion (i.e., moving only the facial muscles). An in-scanner camera monitored participants during performance of the task. Prior to image processing, a standardized multilayer quality control was performed by experienced research staff, and a custom dashboard was used to track key scan quality metrics (
https://github.com/TIGRLab/dashboard).
fMRI Data Preprocessing
Anatomical T1 MR images were processed using FreeSurfer (version 5.3) to generate individualized gray matter, cerebrospinal fluid, and skull masks. The first four repetition times were removed from fMRI scans to allow for magnetic steady state, data were slice time corrected (with the FMRIB Software Library [FSL] slicetimer), and the Analysis of Functional Neuroimages (AFNI) software suite was used to deoblique, deskull, and motion correct the scans. Time series outliers were attenuated via AFNI’s 3Ddespike and echo planar imaging (EPI) data were normalized for intensity. A linear transformation was performed to register the EPI to the T1 data, and a nonlinear transformation (FNIRT) was performed to move the EPI data onto the cortical surface. Data on the cortical surface were then smoothed in two dimensions using an 8-mm Gaussian smoothing kernel.
Statistical Analyses
General linear model analysis.
A first-level general linear model analysis was performed with SPM12 using the canonical hemodynamic response function. Neutral, happy, sad, angry, and fearful faces and the fixation cross were modeled as events. A series of noise regressors was included in the analysis. Thirty-six motion parameters were calculated, including the six basic parameters (three translations and three rotations), the lags of these six parameters, the first derivatives of these six parameters, and the square of each of those 18 values. A principal component analysis was performed on those 36 regressors, and the first three components were entered into the model. We also included the framewise displacement for each repetition time, and the first three principal components of the time series in the white matter voxels and the cerebrospinal fluid (using a custom CompCorr function implemented in Python). Emotional faces (happy, sad, angry, and fearful) in the imitate block were contrasted with emotional faces in the observe block. Beta and t-maps were calculated for each participant for this contrast, and a second-level group analysis was run separately for each site and group. Group analysis was conducted using threshold-free clustering (
31) with the PALM package. A threshold for the data was set at p<0.05, family-wise error corrected.
Hierarchical clustering of fMRI data.
Hierarchical clustering was performed to identify data-driven groups of participants who shared similar patterns of neural circuit activation. For each participant, the first-level t-map of cortical vertices was transformed into a vector representing the spatial pattern of activity in each participant (a spatial series). T-maps were used rather than beta maps, because t-values incorporate variance within the signal, and thus de-weight noisy voxels and potentially spurious signals. Ward’s method was used, with Euclidean distance. The cluster analysis was implemented in MATLAB (release 2014b) via the linkage and cluster functions. The number of clusters was determined using cluster stability analysis (
32), defined as local minimums for instability across a range from two to 10 clusters.
Euclidean distance calculations by site and diagnosis.
To assess differences across sites, we calculated the Euclidean distances between participants. A separate one-way analysis of variance (ANOVA) was calculated for participants from each site comparing their distance to participants from all sites to discover any site-related bias. To assess whether cluster membership was more important than diagnostic category in the clustering solution, we calculated each participant’s average distance to all other participants in the same diagnostic group (e.g., schizophrenia spectrum disorder, healthy control) and the average distance to all other members of their cluster (collapsed across diagnoses). These numbers were subtracted, such that a positive value indicated that a participant was more similar to members of his or her own data-driven cluster than to members of his or her own diagnostic group.
Social cognitive and neurocognitive scores.
Given our previous findings that both lower-level and higher-level social cognitive task performance were related to neural activity during the imitate/observe task (
17), we included both levels of tasks in our social cognitive battery. All test scores were Z-transformed using the mean and standard deviation from control participants. All scores greater than three standard deviations above or below the control mean were excluded. To minimize statistical comparisons, a principal component analysis (RStudio 3.1.2; no rotation) was performed on the six social cognitive scores and the six MATRICS cognitive domains. ANOVAs were conducted for site, diagnosis, and diagnosis-by-site interactions. ANOVAs were used to detect differences in principal component analysis scores between clusters as well as to assess the cluster-by-diagnosis interaction. Additional characteristics were examined between clusters, including site, diagnosis, and sex, using Pearson’s chi-square test, as well as age, education, and motion (framewise displacement) during fMRI scans, using one-way ANOVAs.
Results
SPINS Discovery Study
Sample characteristics.
A total of 185 participants were recruited into the SPINS study. Six scans were excluded because of incomplete brain coverage (N=2), extreme motion (N=2), or potential clinical findings (N=2), which left a final study sample of 179. Demographic information and clinical ratings across sites are presented for the SPINS discovery sample in
Table 1 and for the independent replication sample in Table S1 in the
online supplement.
Cross-site group activations.
A group general linear model across all three sites demonstrated increased activity (imitate > observe) in the motor/premotor cortex and regions associated with the simulation network (
17,
23–
25). A group analysis for each site is shown in Figure S1 in the
online supplement. Significant differences were found in motion (mean framewise displacement) across sites (F=6.4, df=2, 173, p<0.001) and diagnostic groups (F=16.3, df=1, 173, p<0.001), but no site-by-group interaction was observed (F=2.1, df=2, 173, p=0.12) (see Figure S2 in the
online supplement).
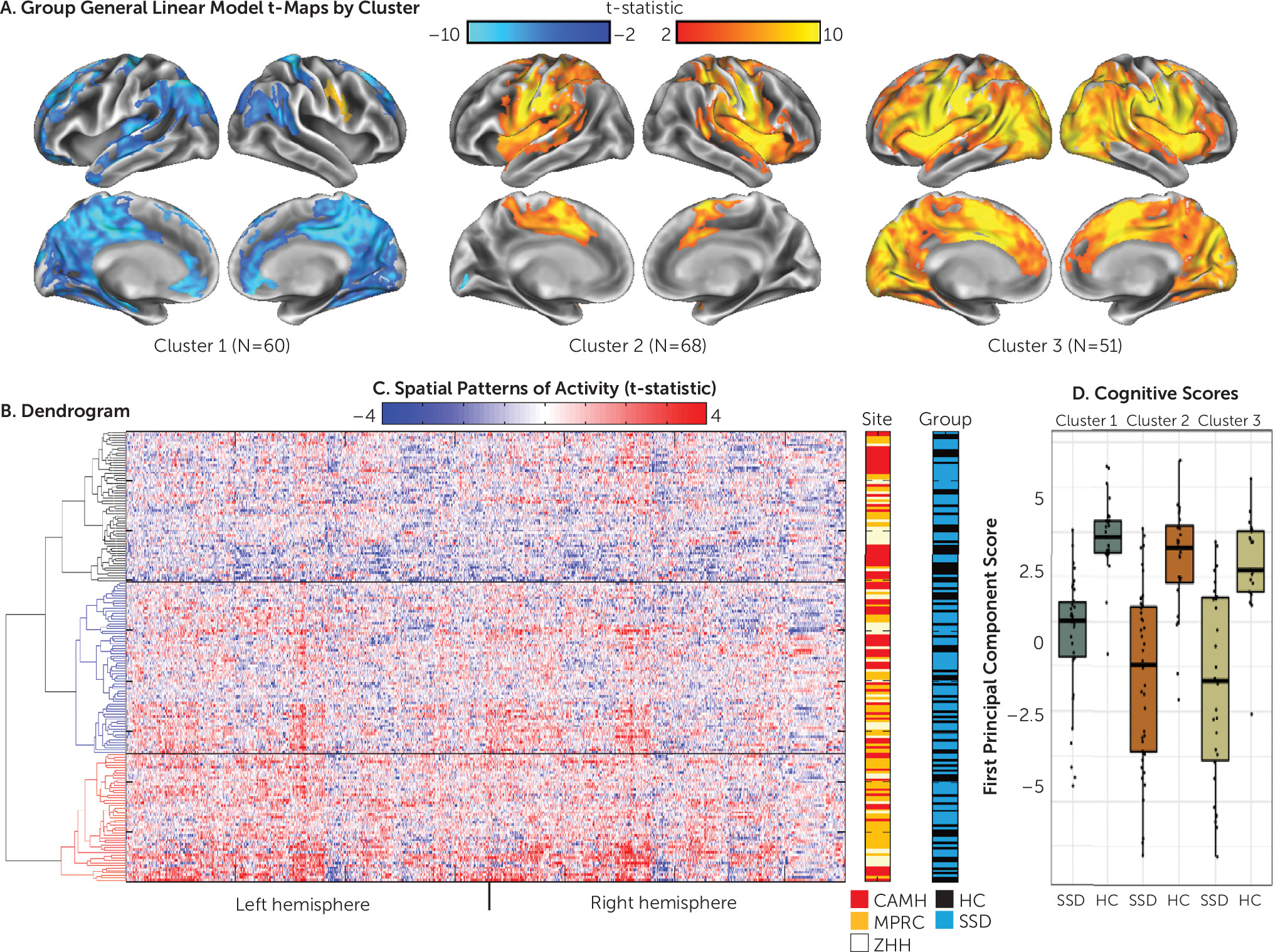
Hierarchical clustering.
The cluster stability analysis indicated a three-cluster solution (
32). A group analysis was then conducted using participant data from each of these three clusters (
Figure 1). In the examination of the patterns of activity within each cluster, participants in cluster 2 appeared to be “typical activators,” with a pattern of activity similar to that shown in the group analysis by site (Figure S1 in the
online supplement) and in previous studies of canonical simulation circuitry (i.e., imitate > observe activity was present bilaterally in the pre- and postcentral, supramarginal, medial superior frontal, insular, and superior temporal gyri and in the left superior parietal cortex) (
17,
23–
25). Participants in cluster 3, “hyperactivators,” appeared to have an inefficient pattern of activity. While increased activity was present in the canonical circuit, these participants also showed a far more diffuse and intense pattern for imitate > observe activity that stretched into other cortical regions. Participants in cluster 1, “deactivators,” showed increased activity in the expected right motor/premotor regions, although to a smaller extent than participants in the other two clusters. Large clusters of deactivation, however, were present in regions typically associated with the default mode network and cortico-midline mentalizing network, including the precuneus, angular gyrus, superior frontal gyrus, posterior cingulate, rostral anterior cingulate, orbitofrontal cortex, and the left and right superior parietal cortex. Examination of the main effects contrast from each cluster (see Figure S3 in the
online supplement) demonstrated that the differing patterns of activity between clusters (e.g., deactivation in cluster 1 and hyperactivity in cluster 3) were driven by differing patterns of activity in the imitate condition compared with the observe condition. A group comparison was also calculated between clusters, demonstrating widespread differences in activity among all clusters (see Figure S4 in the
online supplement).
Cluster characteristics.
The demographic and clinical characteristics for the members of each cluster from the SPINS sample are presented in
Table 2. The clusters did not significantly differ by diagnosis (schizophrenia spectrum disorder or healthy control), site, age, or education or by clinical ratings among the participants with schizophrenia spectrum disorders in each cluster. A significant difference was observed in sex distribution across clusters (χ
2=8.612, df=2, p=0.013) and in mean framewise displacement among clusters (F=5.057, df=2, 176, p
=0.007). To confirm that the distinct patterns of activity among clusters were not due to motion effects, we excluded participants with high motion (mean framewise displacement >0.4 mm; N=20) and recalculated the group analyses by cluster. With these high-motion participants removed, no significant differences in framewise displacement were observed (F=1.53, df=2, 156, p
=0.22), and the patterns of activity in each cluster were unchanged (see Figure S5 in the
online supplement).
Euclidean distance by site and diagnosis.
No differences were observed in the Euclidean distance among participants within or across sites (all one-way ANOVAs, calculated for each site, were p>0.4), demonstrating no systematic biases in the overall pattern of activity across sites. The mean Euclidean distance between participants and other members of their cluster was significantly lower than the mean distance to their diagnostic group (t=14.0, df=178, p=1.3×10−30), demonstrating that participants showed greater similarity to their cluster than to their diagnostic category.
Cognitive performance.
Three SPINS participants were excluded as outliers (scores greater than three standard deviations from the control mean). Descriptive statistics for cognitive scores across all social cognitive and neurocognitive tests in the SPINS sample are presented in Table S4 in the
online supplement. A single principal component accounted for the majority of variance in the principal component analysis for the social and neurocognitive scores (55% of variance explained); the second component accounted for 10.2%, with all scores loading on the first principal component. The ANOVA examining diagnosis by site showed a main effect of diagnosis (F=104.8, df=2, 177, p<0.001; healthy control > schizophrenia), an effect of site (F=4.0, df=2, 177, p=0.019), and a nonsignificant group-by-site interaction (F=2.7, df=2, 177, p=0.066). To confirm that the factor structure was similar across clusters, a principal component analysis was repeated separately for each cluster; factor loading onto the first principal component was strongly correlated across the clusters (r>0.95). ANOVA results for cluster and the cluster-by-diagnosis interaction revealed significant differences by cluster (F=5.32, df=2, 170, p=0.006) but no cluster-by-diagnosis interaction (F=0.4, df=2, 170, p=0.66) (
Figure 1D), with cluster 1 deactivators performing better than participants in clusters 2 and 3 (post hoc t tests p<0.05).
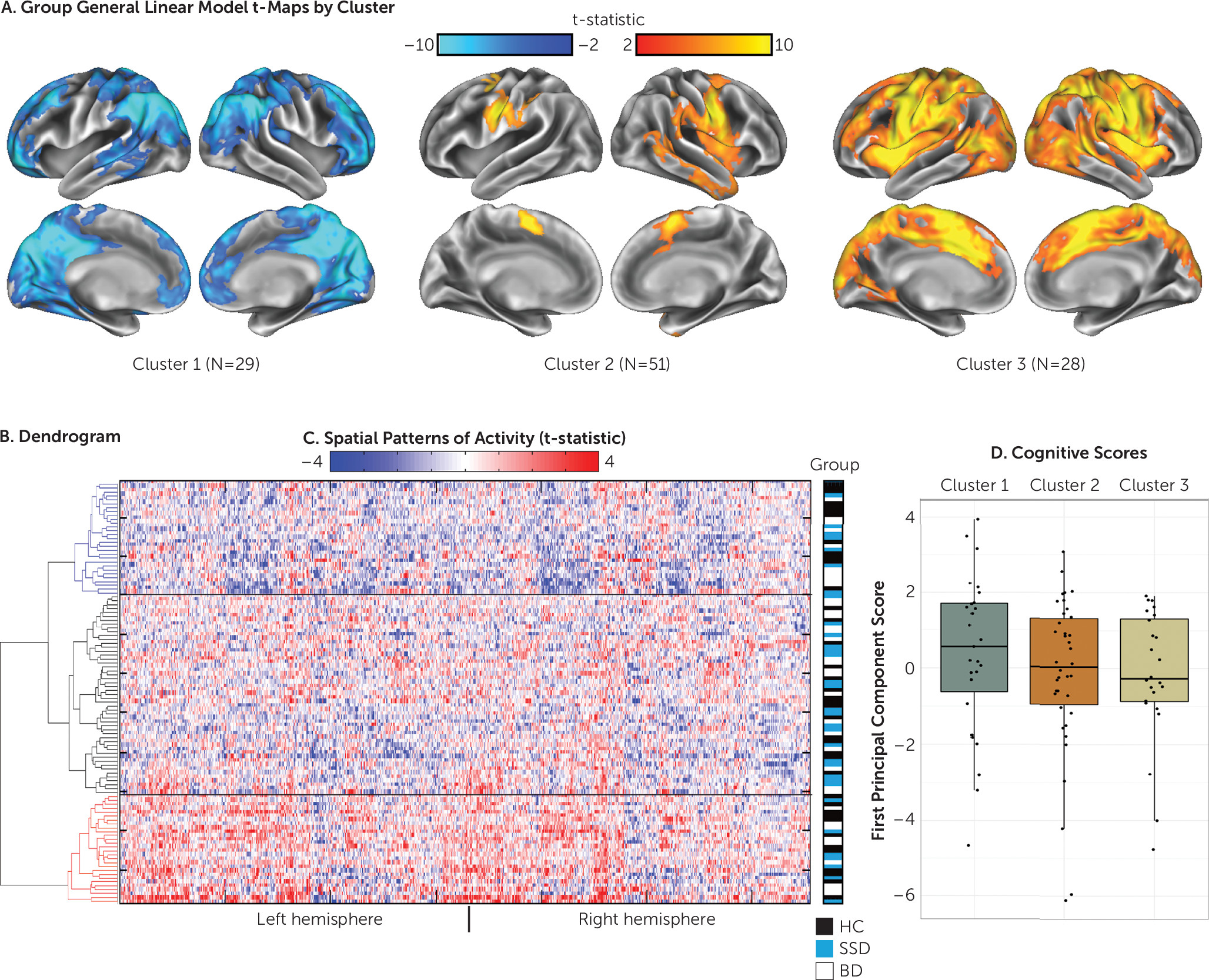
Independent Replication Study
When hierarchical clustering was conducted on the independent sample (N=108, at the Centre for Addiction and Mental Health only; 32 with schizophrenia spectrum disorders, 37 with euthymic bipolar disorder, 39 healthy control participants), the three emergent clusters showed patterns similar to those of the discovery sample (
Figure 2). A behavioral analysis was conducted using the first principal component of the combined social cognitive and neurocognitive data (recalculated independently for this sample). Complete social cognitive and neurocognitive data were available for 89 of the 108 participants. While behavioral scores did not significantly differ between clusters, the pattern was identical to that of the larger discovery sample, with deactivators (cluster 1) showing the highest mean scores (
Figure 2D). Cluster characteristics are shown in Table S3 in the
online supplement. No significant differences were found in clinical ratings, demographic characteristics, or motion between clusters.
Discussion
Using a hierarchical clustering approach in the SPINS sample, we were able to identify three groups of individuals with distinct patterns of neural circuit engagement during imitation versus observation of emotional faces. These three groups also showed differences in social cognitive and neurocognitive test performance. Similar groups were identified in a large replication sample that also included people with euthymic bipolar disorder, suggesting that these patterns represent replicable activation profiles, are generalizable across diagnostic groups, and are unrelated to scanner or site.
We hypothesize that cluster membership represents differences in the underlying “strategy” used by different participants to perform the task (e.g., suppressing activity in task-irrelevant systems or activating a more extensive set of regions to compensate for local deficiencies) (
6–
8). Our recent work (
17) suggests that poor social cognitive performers may make greater use of mentalizing regions when imitating emotional faces, as a compensation for or a consequence of poor social processing. Consistent with this, the hyperactivators, who showed reduced social cognitive and neurocognitive test performance relative to deactivators, activated a more extensive range of both the simulation and mentalizing circuits. It is possible that hyperactivators are compensating for inadequacies within their simulation circuit by engaging extended regions. A similar phenomenon is observed in healthy aging, in which local reductions in neural efficiency and flexibility may be compensated for with greater activity in extended neural circuits, including increased bilateral responses (
33).
The cluster 1 deactivators showed a distinct pattern of neural activity, including extended patterns of suppression during the imitate block, and the best social cognitive and neurocognitive test performance. Some of the regions showing suppressed activity included default mode regions (e.g., the precuneus) and regions implicated in theory of mind, such as the medial prefrontal and posterior cingulate cortex (
34). Increased suppression of default mode activity, representing a reduction in task-irrelevant processing, has been previously linked with better neurocognitive test performance (
35). These deactivators also showed the lowest level of activity in the simulation circuit. Several studies have shown that activity in these regions is reduced when imitating well-known as opposed to novel actions (
36,
37), and neural efficiency in the form of decreased activity in local regions has been linked to general cognitive ability (
38). This pattern of activity and the higher cognitive scores suggests that these participants may be making use of an optimized pattern of brain activity while performing the task. This raises an interesting possibility: can social cognition in typical activators and hyperactivators be improved if an individual’s pattern of brain activity is shifted toward this apparently optimized configuration (
39)?
Using a data-driven approach, we found that the replication sample, which also included participants with euthymic bipolar disorder, exhibited the same three patterns of neural circuit activity during the imitate/observe task. Similar to findings with the discovery sample, we found that cluster membership was unrelated to diagnostic category. While a small number of studies have raised the possibility of simulation circuit dysfunction in bipolar disorder (
40), to our knowledge this is the first study to include such participants with this imitate/observe paradigm. The independent sample provides critical validation of the importance of considering individual variation in the pattern of activation during the performance of the imitate/observe task, not just in schizophrenia spectrum disorders, but across disorders and in healthy control participants. The neural circuit engagement of these subgroups of participants may have been missed in studies using a case-control approach (
9,
10). Within both the discovery and replication samples, approximately 50% of participants fell outside the cluster of typical activators. This finding highlights the importance of considering individual variation within neuroimaging data (
4) and the risk that studies with small sample sizes may not identify representative patterns of activity.
Some limitations of this study should be acknowledged. First, while significant differences in cognition between clusters were observed, substantial overlap remained, and several participants with schizophrenia spectrum disorders among the deactivators were still impaired. Second, in clustering approaches, participants are separated into discrete and categorical groupings. Our group maps, however, suggest a gradation of responses, ranging from a more efficient neural strategy (i.e., deactivators) to a more inefficient neural strategy (i.e., hyperactivators). Finally, motion differences were observed among cluster groups in the discovery sample, which were no longer present after participants with a framewise displacement >0.4 mm were excluded. Although we conducted a number of a priori and post hoc processing procedures, these approaches can only minimize the potential impact of motion as a confounding variable. The most powerful argument against motion as an explanation of our clustering results is the replication sample, in which we achieved the same results as in the SPINS discovery sample, even though no motion difference was present among cluster groups.
After we excluded the participants with the greatest motion, the findings remained unchanged. Motion was also negatively correlated with cognition (
41), suggesting that it is difficult to separate the full range of behavioral functioning from motion. To compensate for this potential problem, we took a multipronged approach that included participant training, data quality control, and modeling of potential confounders such as motion. Such motion effects, however, may be an unavoidable characteristic of the data when ensuring that a full range of behavioral performance is included in a study.
We identified three replicable patterns of brain activity in response to a social imitate/observe task. Of note, the deactivators and hyperactivators showed patterns of activity outside the canonical fronto-parietal simulation circuit present in the typical activators, which were not present in the group-by-site analysis. Standard group approaches would have missed this critical information on how individual brains can perform a task (
11). Furthermore, participants did not cluster by site or diagnosis, but participants showed greater similarity to members of their own cluster than to other members of their diagnostic group. This result calls into question the implicit assumption in case-control designs that groups are homogeneous but distinct from each other. Our results are more consistent with the RDoC framework (
42), as we have demonstrated a gradient of neural efficiency to inefficiency mirroring better to poorer cognitive performance, respectively. On the basis of these specific patterns of neural engagement, targeted interventions can be tested to enhance social cognitive or neurocognitive test performance in people with psychiatric disorders. Such patterns of brain function may represent putative circuits that can be directly probed via brain stimulation and can be objectively measured in the scanner as a treatment response marker in people with psychiatric disorders, complementing performance outside the scanner or self-report.
Acknowledgments
The authors thank Joanna Collaton, Jessica D’Arcey, Shelley Grady, Adriana Halaby, Jef West, Rebecca Ruiz, John Fitzgerald, Kathryn Rhindress, Kristin Minara, Tracy A. Giordonello, Lara Prizgint, Simran Kang, Taylor Marzouk, Sana Ali, Bernie Kompancaril, Christopher Morell, Danielle Beech, Darren Phane, George Nitzburg, Ivana De Lucia, and Natasha Bennett for collecting behavioral and neuroimaging data; Nancy Lobaugh for managing and maintaining the consistency of the Centre for Addiction and Mental Health MRI equipment; and Steve Hawley, Jon Pipitone, Dawn Smith, John Cholewa, and Tom Wright for data management.
Acknowledgments
The authors acknowledge the contributions of the remaining members of the Social Processes Initiative in Neurobiology of the Schizophrenia(s) (SPINS) group: Will Carpenter, Jen Zaranski, Eric Arbach, Sharon August, Peter Kochunov, Peter Kingsley, Xiangzhi Zhou, Sofia Chavez, Gary Remington, Judy Kwan, Christina Plagiannakos, Mikko Mason, Marzena Boczulak, Dielle Miranda, Philipp Homan, and Michael Green.