Attention deficit hyperactivity disorder (ADHD) is a common, highly heritable (
1,
2) neurodevelopmental disorder with a complex and heterogeneous pathophysiology. Pathways toward disease are hypothesized to be mediated by alterations in diverse brain networks (
1). A recent neuroimaging mega-analysis (
3) reported subtle but consistent differences in volumes of subcortical brain regions and intracranial volume (ICV) in ADHD, across diverse cohorts worldwide: compared with healthy control subjects, patients with ADHD showed decreased ICV and volumes of the nucleus accumbens, amygdala, caudate nucleus, hippocampus, and putamen. How such alterations contribute to the disease phenotype is still poorly understood. However, brain volume alterations are also present, on average, in unaffected relatives of patients with ADHD (
4,
5), and both ADHD and brain volumes have high heritability (60%−70% [
2,
6] and 70%−90% [
7], respectively). This suggests that genetic variants underlying ADHD pathophysiology may also influence brain volume variation. Recently, the first genome-wide significant loci for ADHD were identified, and a single-nucleotide polymorphism (SNP)-based heritability of 20.16% has been reported (
8). In the present study, we investigated the genetic covariance between ADHD risk and structural brain phenotypes; we set out to determine whether common genetic variants are shared between ADHD risk and brain volumes that are found to be altered in ADHD (ICV and volumes of the nucleus accumbens, amygdala, caudate nucleus, hippocampus, and putamen). These volumes were selected to focus on the most robust imaging phenotypes in ADHD (
3).
Genome-wide association studies (GWASs) have identified 10 genome-wide significant loci associated with hippocampal volume (
7,
9–
12), eight with ICV (
7,
9,
13,
14), four with putamen volume (
7), and one with caudate volume (
7). These variants explain only a small fraction of the heritability of these brain volumes (
7,
9–
11,
13). Recently, Franke and colleagues reported on a battery of statistical tools (
15) to comprehensively examine genetic overlap between brain volumes and risk for brain disease at the genome-wide level and at the level of individual risk variants, using schizophrenia as an example. Here, we applied a similar set of methods to identify and dissect genetic sharing between ADHD and brain volumes implicated in ADHD based on the latest mega-analysis (
3).
Discussion
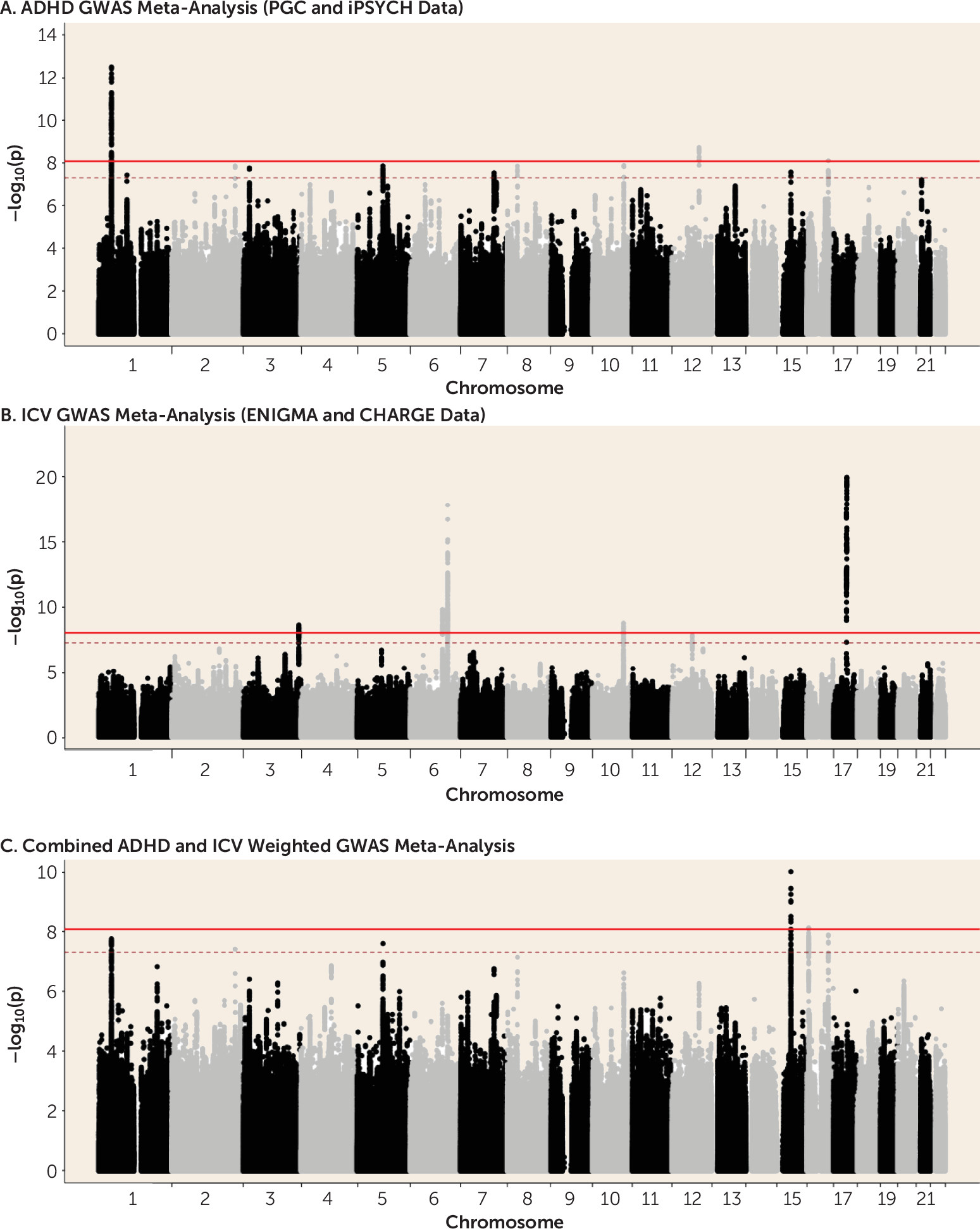
In this study, we set out to investigate genetic covariance between ADHD risk and structural brain phenotypes. We found significant, though modest, genetic covariation between ADHD risk and brain volumes, both on global and gene-wide/single variant levels. On the global level, significant negative genetic correlation between ADHD and ICV was demonstrated. The direction of effect was supported by SNP effect concordance analysis. Our ICV finding was highly consistent across approaches and in the expected direction, given the previous observation that patients with ADHD have smaller ICV relative to control subjects (
3). For most subcortical brain volumes, pleiotropic effects were also found. On the single variant and gene-wide levels, meta-analyses found significant loci associated with both ADHD risk and brain volumes. We identified
SEMA6D,
KIZ, and
MEF2C as potential key loci contributing to both ADHD risk and ICV, and exploratory gene-set analysis revealed association of ADHD–ICV overlap with variation in neurite outgrowth genes.
A reduction of subcortical brain volumes and ICV is not unique to an ADHD diagnosis, but is also seen in depression and bipolar disorder (
26,
27). However, genetic correlation between ADHD and ICV shows some specificity to this disorder, as it was not found in studies of other mental disorders, including schizophrenia (
14,
15), major depressive disorder (
28), and autism (
14), using similar methods. On the other hand, power issues should not yet be discarded as a reason for the lack of finding genetic correlations, despite the large sample sizes, and results of a recent study found that schizophrenia and brain structure volumes share genetic risk factors using a conditional false discovery rate analysis (
29).We also observed significant pleiotropy between ADHD and amygdala, caudate nucleus, hippocampus, and putamen volumes. This global genetic covariation was substantiated by local effects, which we observed in the weighted cross-phenotype meta-analyses. In addition to ICV, variation in the volumes of the caudate nucleus and putamen also showed significant genetic concordance with ADHD. However, whereas results for ICV were in line with our expectation, concordant effects for ADHD and nucleus accumbens and caudate nucleus volume were counterintuitive (ADHD patients have smaller volumes for these structures [
3]), suggesting a reverse or more complex pattern of causation. It should be noted that the subcortical regions were corrected for ICV phenotypically, so that their genetic correlation was limited.
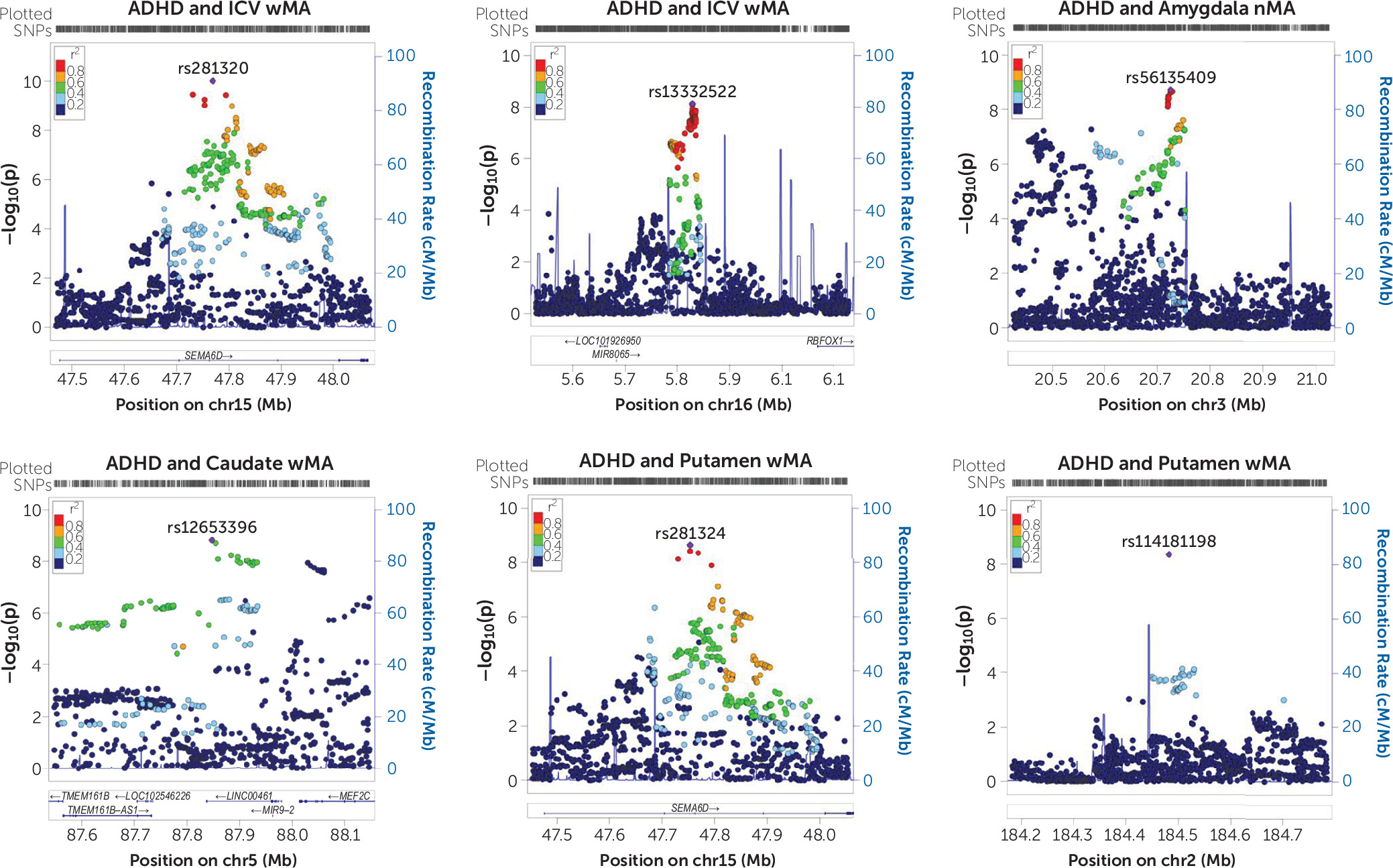
On the single variant level, we only had tools available to perform a meta-analysis by looking at concordant effects (
8), and we therefore had to ignore locus-specific discordant effects. Still, the strongest association of single genetic markers was observed for ADHD and ICV, and additional associations were identified for ADHD and subcortical volumes. The weighted meta-analysis of ADHD and ICV found two potentially pleiotropic loci. One of those was
SEMA6D, coding for the semaphorin 6D, a transmembrane molecule important for maintenance and remodeling of neuronal connections (
30). Animal studies have shown that it acts as ligand for PlexinA1, which is involved in neuronal development in the spinal cord (
31). Together with the gene-based cross-trait result identifying the
MEF2C gene and the findings in the exploratory gene-set analysis, our findings suggest that neurite outgrowth dysregulation may act as a neural mediator of ADHD. Dysregulation of neurite outgrowth may pose a more general genetic risk for psychopathology, as it has been shown to be involved not only in ADHD (
22,
32) and the hyperactive/impulsive symptom domain of ADHD (
33) but also in dyslexia (
34) and autism (
35). We also found the
SEMA6D locus in the cross-phenotype meta-analysis for ADHD and putamen volume, even though this volume had been corrected for ICV, suggesting that genetic variation in
SEMA6D may influence specific brain regions to varying extents. In line with our gene-set association results, a recent study using data from the UK Biobank mainly found associations between MRI measures and genes involved in brain development and plasticity (
36). Since most of these genes have also been demonstrated to contribute to different psychiatric and neurodegenerative disorders (
36), specificity of our findings for ADHD requires additional investigation.
Our results in this study raise a number of questions concerning the way alterations in the brain mediate etiological risk pathways in ADHD. The first question is about the role of cognitive performance in this relationship. ADHD and ICV were recently shown to be genetically correlated with intelligence (ADHD is negatively genetically correlated with IQ [r
g=−0.37, p=2.21×10
−2], and ICV is positively correlated with IQ [
37] [r
g=0.29, p=3.44×10
−4]). Similarly, educational attainment is linked to both ICV (r
g=0.34, p=1.2×10
−6) and ADHD (r
g=−0.54, p=1.44×10
−80), as well as to IQ (
8,
38). It may therefore be possible that the genetic link between ADHD and ICV is mediated by IQ and its proxies. We attempted to test this—in the absence of IQ and educational attainment data to correct for in the ADHD GWAS meta-analysis—using a sign test based on data from the recent large intelligence GWAS meta-analysis (
16). Here, we found an overrepresentation of opposite-direction effects of ADHD–ICV SNPs in the intelligence GWAS meta-analysis data, suggesting that intelligence may indeed play a role in the ADHD–ICV overlap. However, of the 43 SNPs included in the analysis, only 15 (34.8%) were nominally significantly associated with intelligence, suggesting that the genetic link between ADHD and ICV is additionally driven by intelligence-independent effects. More in-depth research will be needed to fully understand the role of intelligence in the ADHD–ICV overlap in the future; it may occur upstream and/or downstream of our correlation finding. Second, the degree of sharing observed was statistically modest. At first sight, this seems to be inconsistent with the general hypothesis that ADHD is a genetic-based brain disorder. However, there are a number of possible explanations for this modest sharing. We examined brain structure at a gross anatomical scale; compared with more precise methods, such as voxel-based or surface-based morphometry, atlas-based brain segmentations may be too coarse to identify subtler volumetric differences. Notably, for the type of imaging genetics analyses described here, we were strongly dependent on the availability of GWAS data for brain phenotypes. These GWAS data have to be derived from large-scale studies to allow sufficiently powered analyses. Such data are, so far, available only for subcortical volumes and ICV, published by the ENIGMA and CHARGE consortia. The sample sizes of the few voxel-wise GWASs available to date are not large enough to offer sufficient statistical power for the genome-wide approaches presented here. Moreover, it may be more informative to study structural and functional connectivity measures. In addition, as pointed out previously (
15), the limited SNP heritability of subcortical brain volumes further challenges the identification of genetic overlap, and more highly powered studies of brain phenotypes may lead to higher estimates of overlap. Also, the field may advance by applying more sophisticated imaging approaches to imaging genetics studies, such as redefining imaging phenotypes through dimension reduction approaches (
39). Finally, it is also possible that some of ADHD’s association with reduced brain volumes is driven by environmental effects, either independently or in interaction with genetic factors (
1).
Previous brain imaging genetics studies in ADHD have mainly focused on single genetic variants and have been hampered by limited sample sizes (
40). This study combined the largest data sets available to investigate the genetic overlap between ADHD and brain volumes by using a complementary battery of statistical methods. Nevertheless, some limitations apply. First, this study focused on a limited set of mainly subcortical MRI measures, and future work should be extended to cortical regions and connectivity measures, once large-scale GWAS meta-analysis becomes available (
4,
41). To support highly sophisticated imaging genetics analyses, which can provide granular information on specific circuits of relevance, there is also an increasing need for large-scale imaging cohorts with raw imaging and genetic data that allow maximal flexibility in the application of analytic methods. Second, for the cross-phenotype GWAS meta-analysis, we used a recently described weighted meta-analysis method (
8). However, we observed that with low and moderate genetically correlated phenotypes, the association signals generally did not improve over a naive meta-analysis performed without adding additional weights (see Figure S10 and Table S22 in the
online supplement). In addition, we could only investigate concordant SNP effects with this method. Third, generalization of our findings to other ethnicities should be assessed in future studies. Fourth, it is possible that this study underestimated genetic correlations, as we did not take into account the known role of rare and structural variants in the genetic architecture of ADHD (
42,
43). Future studies investigating heritability and genetic correlation could also benefit from including variants with low minor allele frequency and in low-LD regions, which may reveal stronger relationships between ADHD and brain volumes.
To our knowledge, this is the first study to show significant global and single gene/variant level genetic correlations derived from polygenic overlap between ADHD and brain volumes. The modest genetic overlap between ADHD and variation in brain volumes is consistent with models implicating alterations in brain structure in ADHD-related genetic risk pathways and provides new hypotheses about neurobiological mechanisms involved in ADHD.