Anxiety disorders are among the most common psychiatric disorders and can become chronic (
1,
2). They affect more than one in four individuals at some point in their life and are generally more prevalent in females than in males (
3,
4). Individuals suffering from anxiety disorders have impairments in psychosocial functioning and quality of life (
5), and when severe, anxiety disorders can be disabling. Children are especially affected by pathological anxiety, as anxiety disorders are among the earliest psychiatric illnesses to develop (
2), affecting up to 20% of youths (
6–
8). Additionally, anxiety symptoms in children are often comorbid with other conditions, and they are a strong predictor of the subsequent development of other anxiety disorders, affective disorders, and comorbid substance abuse (
2). While early treatment has the potential to prevent the lifelong suffering and psychosocial dysfunction associated with these disorders, current treatments for childhood anxiety disorders, such as cognitive-behavioral therapy and selective serotonin reuptake inhibitors, are suboptimal. Many children fail to respond to these treatments, and those who do respond have relatively high rates of relapse (
9). Establishing a better understanding of the pathophysiology of childhood anxiety disorders will facilitate the development of novel, more effective treatments (
10).
Despite the prevalence and importance of childhood anxiety disorders, few studies have focused on characterizing the neural alterations that underlie the early manifestations of these disorders (
11). Current knowledge of the pathophysiology of anxiety disorders is mostly derived from studies of adults, and a few studies have examined adolescent and preadolescent patients with anxiety disorders (
11). Previous work revealed that anxiety disorders are associated with hyperactivation of the amygdala and insula in response to negative emotional stimuli (
12–
14), and recent work from our group has shown that amygdala activation is significantly higher in preadolescent children with anxiety disorders when confronted with uncertain conditions (
15). We, as well as others, have also reported decreased anxiety-related functional coupling between the amygdala and regulatory prefrontal cortical regions such as the anterior cingulate cortex, orbital frontal cortex, dorsolateral prefrontal cortex, and medial prefrontal cortex (
16–
19). Decreased anxiety-related functional coupling between the amygdala and the prefrontal cortex is evolutionarily conserved, as we demonstrated similar findings in a rhesus monkey model of early-life anxiety (
18).
Complementing these findings, diffusion tensor imaging (DTI) studies in patients with anxiety disorders report altered structural connectivity between temporal lobe and prefrontal cortical regions, revealing that fractional anisotropy (FA) in the uncinate fasciculus (UF) is reduced in patients with anxiety disorders (
20–
25). These studies focused predominantly on adults with anxiety disorders, with only one study including adolescent patients (
25). The UF is highly relevant to anxiety and emotion regulation, as it connects structures that are crucial to affective processing, such as the amygdala, entorhinal/perirhinal cortices, and parahippocampal gyrus, to frontal regions, including the anterior prefrontal cortex, orbital frontal cortex, ventromedial prefrontal cortex, anterior cingulate cortex, and insula (
26,
27).
While the structural alterations identified in patients with anxiety disorders may reflect the pathophysiology associated with the disorders, it is also possible that they result from illness chronicity, medication exposure, or other nonpathophysiological factors. Studies in medication-naive children have the advantage of examining white matter pathways early in the illness and in the absence of many of the influences that may indirectly affect white matter. Additionally, these studies may inform us on mechanisms underlying childhood risk of developing anxiety disorders and may also point to new early-life treatment and prevention strategies.
In this study, we used DTI to assess white matter integrity in unmedicated preadolescent children with anxiety disorders compared with healthy control children to test the hypothesis that childhood anxiety disorders are associated with alterations in the UF FA. Because of known sex differences in the prevalence of anxiety disorders (
3,
4) and the interest in sexual dimorphism in relation to brain development (
28,
29), we also examined sex differences in this effect. Additional analyses aimed to further characterize these microstructural alterations by investigating three supplementary diffusivity measures as well as interactions with symptom measures and steroid hormones. Beyond the UF, we explored six additional structures for which the relation to anxiety disorders has been less consistent (
20–
25,
30,
31), and we conducted a whole brain voxel-based analysis.
Methods
Participants
For a detailed description of the study methods, see the Supplemental Methods section in the
online supplement. Briefly, diffusion-weighted MRI scans were obtained from a preadolescent sample at two research sites: University of Wisconsin and the National Institute of Mental Health (NIMH). The final sample consisted of 98 children ages 8–12 (50 of them girls) (
Table 1), of whom 46 were control subjects, across the two sites. Control subjects were age- and sex-matched at the group level to the anxiety disorder sample and had no current or past psychiatric illness. Neither the anxiety disorder patients nor the control subjects were currently on any psychotropic medication, and none reported any psychotropic medication usage at any point during their life. Informed assent and consent were obtained from all participants and their parents, in accordance with the institutional review boards of the University of Wisconsin and NIMH. Individuals were compensated for their time and effort.
All participants were interviewed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (
32), which was administered by a trained Ph.D.-level clinical psychologist or a psychiatrist. (See the Supplemental Methods section in the
online supplement for details on interrater agreement.) Beyond diagnosis, children’s symptoms were rated by both the child and a parent on multiple symptom questionnaires (see the Supplemental Methods section).
Data Acquisition and Analyses
DTI acquisition.
For a detailed description, see the Supplemental Methods section in the online supplement. Briefly, brain images at both sites were collected on MRI scanners of the same make and model, with comparable parameters, optimized for diffusion-weighted imaging with 48 directions.
Steroid hormone collection.
See the Supplemental Methods section in the online supplement.
DTI analyses.
For a detailed description, see the Supplemental Methods section in the
online supplement. Briefly, images were corrected for field inhomogeneity and eddy currents, after which the tensors were calculated using a robust tensor estimation. Tensor images of all subjects were coregistered iteratively using nonlinear tensor-based normalization tools and then registered to Montreal Neurological Institute MNI152 space. In this template space, diffusion measures were extracted to quantify local white matter microstructure. Deterministic fiber tractography was performed to delineate tracts of interest, which, besides the UF (
20–
25), included cortico-limbic association pathways previously implicated in anxiety disorders, including the cingulum bundle (
20,
30), the superior longitudinal fasciculus (
20,
24,
25), the fornix (
20), and the inferior fronto-occipital fasciculus (
20,
25). Tracts that have been shown in other studies to have anxiety-related changes were also extracted, including thalamocortical projection fibers in the internal capsule (
20,
25) and interhemispheric commissural fibers of the corpus callosum (
31).
Next, to quantify the microstructure of an entire white matter structure, a weighted mean was calculated per tract for each diffusion measure per subject. Tract-based analyses were performed with average tract measures predicting anxiety disorder patient status. To characterize microstructural changes in these white matter pathways beyond FA, analyses assessed mean diffusivity (MD), axial diffusivity (XD), and radial diffusivity (RD) as well. We also examined the possibility of sexually dimorphic effects relating white matter microstructure to anxiety in children with anxiety disorders. In order to test sex differences in these relations, the interaction between patient status (anxiety disorder, control) and sex (boys, girls) was tested in the same model. Age, sex, and site were included in this model as covariates. Since we had no a priori hypothesis about differences between left and right tracts, the left and right components of each tract were combined into one average.
Analyses were conducted using standard linear regression techniques in RStudio, version 1.0.136 (
33). To control for family-wise error, a Šidák correction for the number of tracts was applied, where m=7 for each white matter tract tested, resulting in a corrected two-tailed significance threshold (alpha) of 0.0073 (α
SID=1−[1−α]
1/m=1−[1−0.05]
1/7=0.0073). The effect sizes of significant tract-based effects were reported using Cohen’s d (
34–
36) to aid in interpretation. Cohen’s d values greater than 0.8 are considered a large effect size, and 0.5 a medium effect (
36). Voxel-based analyses of FA across the brain were performed to test regions within and beyond the tracts that were determined a priori.
Additional analyses, described in detail in the Results section, included age, sex, and site as covariates where applicable and used standard linear regression techniques in RStudio and Python.
Steroid hormone analyses.
See the Supplemental Methods section in the online supplement.
Results
Demographic Characteristics, Symptoms, and Hormonal Measurements
Ninety-eight children, 46 of them control subjects, were included in the final analyses. Children with anxiety disorders and control children did not differ significantly in age (t=0.385, df=94, p=0.70), sex (t=0.184, df=94, p=0.85), or IQ (t=0.888, df=91, p=0.38), and there was no significant difference between groups in physical development as assessed by the Tanner stages (t=−1.499, df=86, p=0.14) (see
Table 1). Age and sex each significantly predicted Tanner score (age: t=8.510, df=86, p<0.001; sex: t=−2.006, df=86, p=0.048), with older children and girls having significantly higher Tanner scores, but the group-by-sex interaction was nonsignificant (p=0.41).
Children with anxiety disorders had significantly higher symptom scores on all clinical scales (anxiety, depression, and attention deficit hyperactivity disorder [ADHD]) compared with control children (p values <0.001) (see
Table 1). No group-by-sex interactions were found for depression and ADHD symptoms. A group-by-sex interaction approached significance for self-reported anxiety (t=1.82, df=89, p=0.072) (see Figure S1 in the
online supplement). Post hoc tests indicated that self-reported anxiety scores were significantly lower for boys compared to girls with anxiety disorders (t=2.49, df=48, p=0.016).
Testosterone level was significantly higher in girls compared with boys (z=−2.57, df=55, p=0.006; for mean values by group for each sex, see
Table 1), an effect that has previously been observed in preadolescent children (
37). Neither estradiol nor cortisol levels differed by sex (p values >0.4). Age was positively related to testosterone (z=3.286, df=55, p=0.006) and estradiol (z=2.538, df=55, p=0.011) levels.
While there were no group differences in cortisol or testosterone levels (p>0.22), an unpredicted significant group effect was observed for estradiol levels, in which children with anxiety disorders had lower levels of estradiol compared with control children (z=2.085, df=55, p=0.037) (see
Table 1; see also Figure S2 in the
online supplement). None of the endocrine measures displayed a significant group-by-sex interaction (p values >0.5).
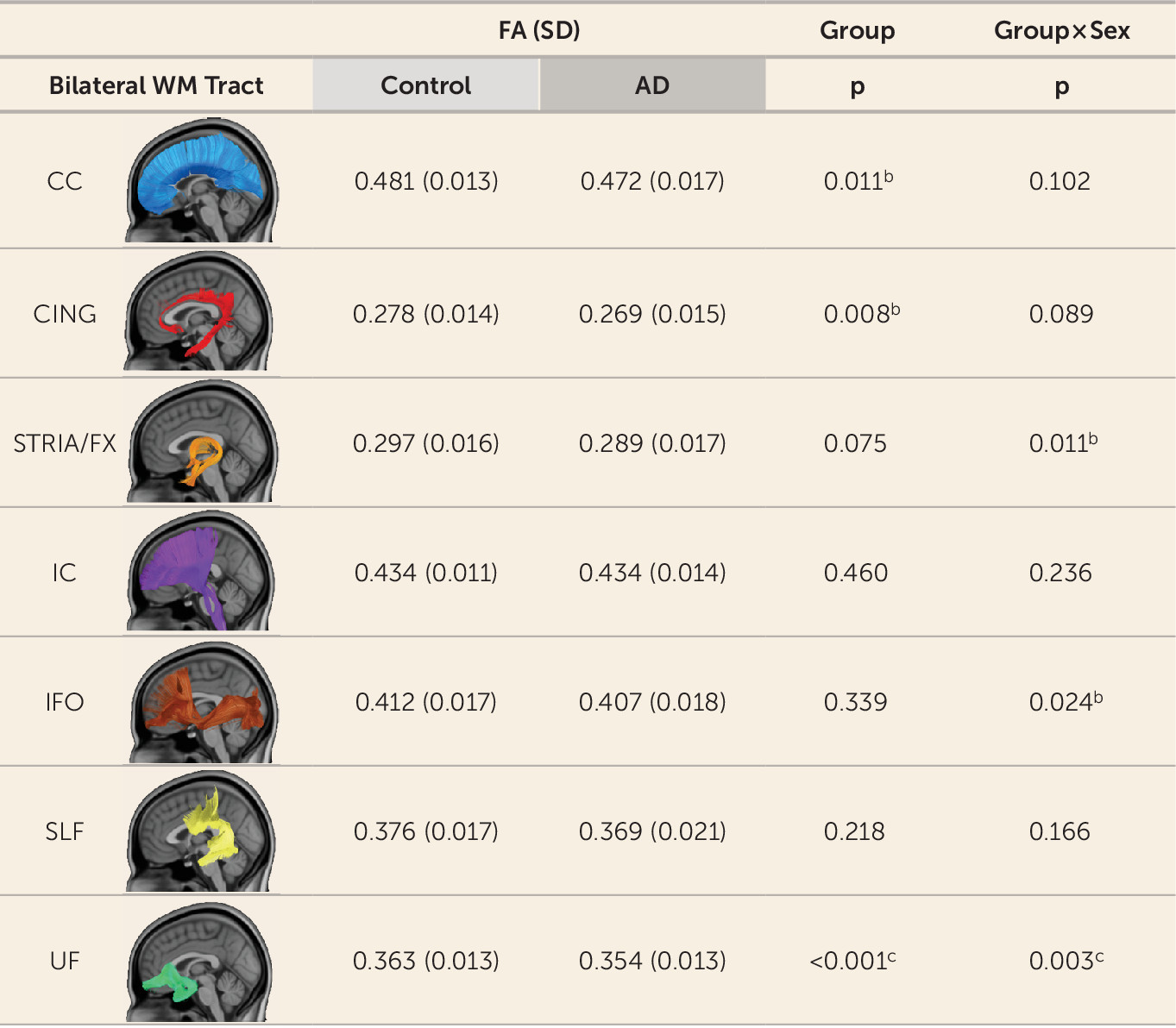
Tract-Based DTI Analyses
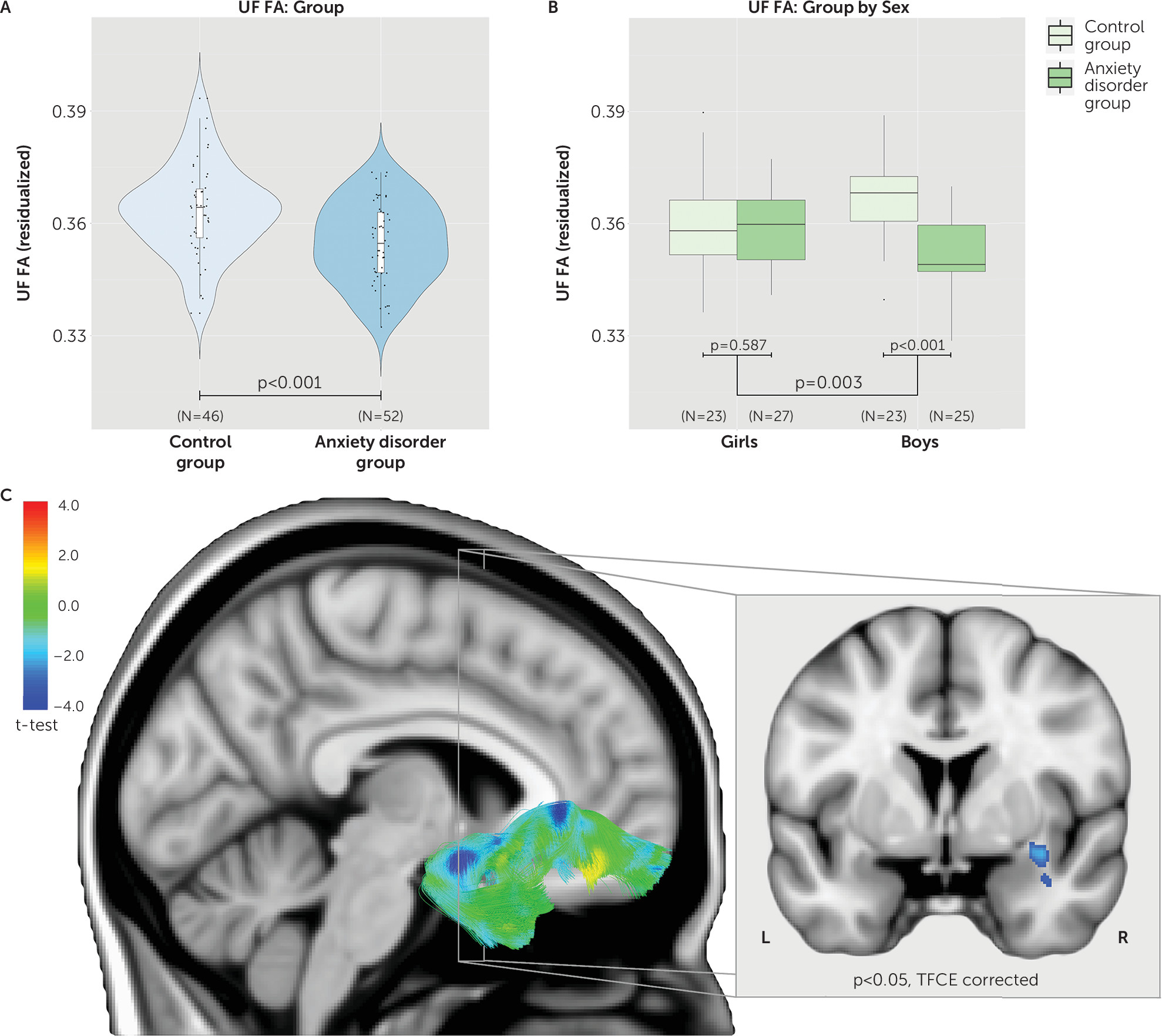
Tract-based analyses were performed on seven white matter pathways, leading to a multiple-comparisons threshold after the Šidák correction of p≤0.0073, and were covaried for age, sex, and site. Results indicated that only the UF tract significantly differed between groups (
Figures 1 and
2). Specifically, children with anxiety disorders had reduced UF FA compared with control children (t=3.650, df=92, p<0.001; d=0.73) (see also
Figures 1 and
2). In addition, the interaction between group and sex was significant for UF FA (t=3.058, df=92, p=0.003). Post hoc tests indicated that boys with anxiety disorders displayed significantly reduced UF FA compared with control boys (t=4.750, df=44, p<0.001) (see
Figure 1), whereas girls with anxiety disorders did not differ from control girls (t=0.547, df=46, p=0.587) (see
Figure 1). To further investigate which of these four groups were different from the rest (girls with anxiety disorders, boys with anxiety disorders, control girls, control boys), we ran a contrast analysis. The results indicated that boys with anxiety disorders were different from all three other groups (t=3.937, df=92, p<0.001).
Other variables that could potentially influence the results include age, comorbid symptoms, and hormonal status. While there was a main effect of age on UF FA (t=2.786, df=92, p=0.006), no significant sex-by-age or group-by-sex-by-age interactions were found (p values >0.28) (see Table S1 and Figure S3 in the online supplement). In relation to anxiety, depression, and ADHD scores, we performed analyses to test for interactions and found that these measures did not affect the findings, such that there were no significant group-by-sex-by-rating interactions (p values >0.375) (see Table S1 in the online supplement). We also performed analyses that examined potential influences of the comorbid categorical diagnoses, ADHD (four girls, two boys) and depression (three girls, no boys). The results remained largely unchanged when these patients were excluded from the analyses (excluding ADHD: group, t=3.592, df=86, p<0.001; group by sex, t=2.502, df=86, p=0.014; excluding major depression: group, t=3.749, df=89, p<0.001; group by sex, t=2.872, df=89, p=0.005). Finally, we tested hormonal status (cortisol, testosterone, and estradiol) in relation to the findings, which demonstrated no significant interactions that could account for the effects (group by sex by hormone level, p values >0.175) (see Table S1).
Diffusivity measures other than FA that were explored, such as MD, XD, and RD, indicated that only UF RD was significantly increased in children with anxiety disorders (t=−2.813, df=92, p=0.006; d=0.56) (see Figure S4 in the
online supplement), and the group-by-sex interaction was nominally (uncorrected) significant (t=−2.007, df=92, p=0.048) (see Figure S4). Several of the other six white matter tracts displayed some nominally significant group effects and group-by-sex interactions for FA, MD, XD, and RD (see
Figure 2; see also Figure S4), although none were significant after correction for multiple comparisons.
Voxel-Based Analyses of FA
Whole-brain voxel-based analyses explored group differences beyond the a priori hypothesized tracts. The results confirmed the observations from the tract-based analyses, demonstrating significantly reduced FA in multiple locations along the UF tract (p<0.05, threshold-free cluster enhancement [TFCE] corrected; see
Figure 1,
Table 2, and
https://neurovault.org/collections/161/). Areas particularly affected included white matter adjacent to the orbital gyrus and white matter in the bend around the lateral fissure between the temporal and frontal lobes. We also detected group differences in the corpus callosum, internal capsule, and inferior fronto-occipital fasciculus pathways (see
Table 2 and
https://neurovault.org/collections/161/). In the corpus callosum tract, alterations were mainly localized in the genu (p<0.05, TFCE corrected; see
Table 2 and
https://neurovault.org/collections/161/). In the left and right internal capsule, significant clusters were found in the area of the postcentral gyrus in the somatosensory cortex (p<0.05, TFCE corrected; see
Table 2 and
https://neurovault.org/collections/161/). In the inferior fronto-occipital fasciculus tract, significant clusters were mainly located in the anterior inferior portions of the right section of this tract (p<0.05, TFCE corrected; see
Table 2 and
https://neurovault.org/collections/161/).
Based on results from the tract-based analyses, we also examined the group-by-sex interaction, which did not reveal any clusters passing TFCE correction at a p threshold of 0.05. However, separate analyses performed in girls and boys revealed significant FA reductions in boys with anxiety disorders compared with control boys in regions that overlap with the UF, corpus callosum, and bed nucleus of the stria terminalis, and no such differences in girls (see Figure S5 and Table S2 in the
online supplement, and
https://neurovault.org/collections/161/).
Discussion
In this study, we examined the microstructural integrity of the UF in unmedicated, preadolescent children with anxiety disorders and also examined potential sex differences. The findings revealed decreased UF FA in boys, and not girls, with anxiety disorders. Tractography analyses of other white matter tracts demonstrated the relative specificity of this finding. The UF is a white matter tract critical for prefrontal–temporal lobe functional integration (
38), and the present data suggest that in boys this tract may be linked to the prefrontal-limbic dysregulation that is associated with anxiety disorders (
20–
25). While UF FA differences have been reported in adolescents and adults with anxiety disorders, we are unaware of any reports that have addressed the issue of sexual dimorphism in patients with anxiety disorders. However, there have been inconsistent reports of sex-related UF FA alterations in individuals with high levels of trait anxiety (
39–
41).
Notably, we demonstrated that these white matter alterations are present early in life and in children who have never been exposed to psychotropic medications. In contrast to studies in adult populations with anxiety disorders (
20), the results from this study can be interpreted without the potential confounders of prior medication exposure or illness chronicity. These findings provide developmental continuity in relation to the previously reported UF FA reductions in adolescents and adults, suggesting that the UF white matter alterations observed in these older populations may have their origins in childhood.
The UF FA differences that we found in boys with anxiety disorders are intriguing and raise the question as to what underlies this sexual dimorphism. We found no evidence that age or sex hormones could account for this finding. Previous studies in children and adults indicate either no effect or a minimal effect of sex on UF FA (
42–
44). Likewise, studies examining sex steroids in pubertal children have found little association between hormonal levels and white matter volume (
45,
46). However, little is known about the specific relation between sex hormones and UF FA. In contrast, age is well known to be associated with FA (
42,
43,
47,
48), and even with the relatively constrained age range that we studied, we found that UF FA increased with age. One possibility that could explain the sexual dimorphism reported here is that the developmental trajectory of UF FA could differ between boys and girls. However, we found no significant age-by-sex interactions, which is consistent with other work (
43,
44), and we found no age-by-sex-by-group interactions. We also assessed levels of the stress hormone cortisol and found no significant influences of cortisol on UF FA that could account for the UF FA reductions in boys with anxiety disorders, or interactions with sex. Because of the episodic nature of the secretion of steroid hormones, it is possible that more frequent sampling would reveal a relation between steroid hormone levels and UF FA.
Most but not all DTI studies of patients with anxiety disorders report reductions in UF FA as well as other white matter alterations (
20–
25,
30,
31,
49,
50). Inconsistencies in the UF FA findings across studies could be due to a variety of factors, including heterogeneity in diagnostic composition of the sample, sample size, and DTI acquisition and processing. We also note that reduced UF FA is not specific to anxiety disorders, as similar white matter alterations have been observed in some studies of individuals with trait anxiety (
39–
41,
51–
54) as well as in patients with affective and other psychiatric disorders (
55,
56).
Our assessment of multiple diffusivity measures allows for a deeper understanding of the nature of the microstructural alterations found in children with anxiety disorders. In addition to reduced FA, boys with anxiety disorders exhibited increased RD. The combination of decreased FA and increased RD is thought to reflect reduced myelination and/or reduced axonal density (
57,
58). Changes in myelination and axonal density are associated with alterations in the speed, timing, and accuracy of information passing through white matter (
59). Because the UF is the major tract connecting medial temporal lobe structures such as the amygdala and hippocampus with the prefrontal cortex, alterations in UF structure are likely to alter information flow relevant to emotion regulation (
26). Although lesions affecting the UF can alter the regulation of anxiety in primates (
60–
62), the extent to which individual differences in UF myelination influence anxiety-related neuronal signaling is unclear. Of note, a recent study in humans demonstrated that individual differences in UF FA are related to individual differences in cognitive-emotional processing (
63).
Although axon myelination is most active early in development, the microstructural integrity of UF continues to increase into adulthood and is one of the last white matter pathways to reach peak FA (
43). Myelin-producing oligodendrocytes are highly plastic throughout adulthood, and oligodendrocyte precursor cells are the major proliferating cell type in adult brains (
64). Evidence is accumulating that myelination occurs dynamically in response to neural activity and can continue throughout adulthood (
59,
65,
66). This white matter plasticity provides a potential mechanism by which targeted therapies could be focused on restoring UF integrity. Interestingly, data in children suggest that aerobic exercise increases white matter integrity (
67), and it is well known that exercise has antianxiety effects (
68). Our data suggest that early-life interventions directed toward increasing UF integrity could be particularly effective in boys in ameliorating the symptoms of anxiety, and also could have a protective impact on the development of white matter connectivity between brain regions critical for adaptive emotion regulation.
Taken together with other studies (
16–
25), these data provide convergent support for prefrontal cortex–temporal lobe dysfunction, which now extends to childhood anxiety. The findings demonstrate early-life alterations in a key white matter tract involved in conveying information relevant to emotion and anxiety regulation. The findings also point to the importance of considering brain-related sex differences before puberty. The data from this study, along with future studies, have the potential to guide the development of novel treatments focused on restoring the adaptive prefrontal regulation of anxiety. Such early interventions support the possibility of using therapies that could reduce or even prevent long-term chronic psychopathology in the treatment of children with anxiety disorders.
Acknowledgments
The authors thank the HealthEmotions Research Institute, the Waisman Laboratory for Brain Imaging and Behavior, the Lane Neuroimaging Laboratory, Neuroscience Training Program.
Supported by NIH grants R21 MH092581 and Intramural Research Program Project number Z-002781.