Drug abuse is strongly transmitted within families (
1) by genetic and familial-environmental influences (
2–
7). Although substantial efforts are under way to identify molecular genetic risk for drug abuse (
8–
12), less attention is being given to familial-environmental transmission.
Within siblings, shared exposures to community risks such as peer deviance (
13–
15) and psychosocial deprivation (
16), inadequate parenting (
17), and family stressors (
2,
18) likely contribute to the familial aggregation of drug abuse. In this study, we explored another mechanism for familial-environmental effects on drug abuse risk: environmental transmission or “contagion.” Although models of contagion for drug abuse have been applied to community settings (
19,
20), they have rarely been tested in families.
Utilizing the precise timing of drug abuse registration from national Swedish registries, we examined contagion models for drug abuse within relative pairs. First, we examined whether risk for drug abuse among offspring increased after registration for parental drug abuse, and then we applied the same method to full siblings and cousins. Next, we explored whether the magnitude of transmission differed in relative pairs as a function of geographic proximity, age difference, sex, older-younger compared with younger-older pairs, and within classes compared with between classes of psychoactive drugs.
Methods
Several Swedish population-based national registers were utilized and were linked by each study subject’s unique identification number. For confidentiality, this identification number was replaced by a serial number. We secured ethical approval from the regional ethical review board of Lund University. Drug abuse was identified through Swedish medical and criminal registries (for further details, see Table S1 in the online supplement). Individuals could have several registrations in the criminal or medical registers. To avoid double-counting, we allowed for a 90-day period after each registration in which a new registration was not counted.
Using the Swedish multigenerational register, we selected all parent-offspring pairs in which the parent (primary case subject) was registered for drug abuse when the offspring (at risk of becoming a secondary case subject) was between 19 and 23 years old. Drug abuse registration begins at age 16, and at least 3 years of data were required to define a stable baseline. An upper limit of 23 years old was selected because offspring rarely cohabit with their parents beyond age 23. We refer to this registration of the primary case subject as the index registration. We also required that individuals at risk of becoming secondary case subjects reside in the same household with the primary case subject at the time of the index drug abuse registration. For individuals at risk of becoming secondary case subjects, we included drug abuse registration for six different time points: within 1–365 days, within 366–730 days, and within 731–1,096 days before and after the drug abuse registration in the index case. For each pair comprising a primary parental case and an at-risk offspring, we matched several control pairs on sex and year of birth of both parent and child, country of birth and neighborhood socioeconomic status of the child, and number of lifetime drug abuse registrations, type of registration (medical or criminal), and educational status of the parent. We matched on these variables both to control for demographic characteristics (
21) and to control for risk of drug abuse among the relatives of affected case subjects (
22).
Control subjects were matched on drug abuse registration of the primary case subject (residing in the same household as the at-risk secondary case subject) that occurred when the putative secondary case subject was between ages 10 and 19, drug abuse registration of the primary case subject (residing in the same household as the at-risk secondary case subject) that occurred when the putative secondary case subject was between ages 0 and 9, drug abuse registration of the primary case subject that occurred before the putative secondary case subject was 19 years old (whether or not residing in the same household, and the registration could occur before age 0 of the secondary case subject), and lifetime drug abuse registration (yes or no) of the other biological parent and drug abuse registration (yes or no) of the other biological parent that occurred when the at-risk secondary case subject was between ages 19 and 23. All of these variables substantially predicted risk for drug abuse in offspring of affected fathers.
The matching procedure ensured that the difference between individuals at risk of becoming secondary case subjects and matched control subjects was that control subjects 19–23 years old did not have a parent with a drug abuse registration while they were residing in the same household with the parent. In particular, case and control subjects were matched for familial or genetic risk. We included a maximum of five control pairs, but pairings with fewer than five control subjects were also included in the analyses (
Table 1). The use of a control group in the analyses was necessary, because there was a yearly increase in drug abuse registrations among individuals ages 19–23 that we otherwise could not have separated from the effect of the index drug abuse registration among the primary case subjects.
We used a difference-in-difference approach to examine the causal effect of drug abuse transmission from parent to offspring (
23). The underlying assumption in the difference-in-difference model is that the trend in drug abuse among individuals at risk of becoming secondary case subjects would have been the same as the risk among matched control subjects in the absence of the index registration of the primary case subject. To validate this assumption, we included the history of drug abuse registrations among individuals at risk of becoming secondary case subjects (and among control subjects) for 3 years before the index drug abuse registration of the primary case subject. A causal interpretation is supported by a similar trend in the risk for drug abuse before the index registration of the primary case subject among both control subjects and individuals at risk of becoming secondary case subjects but a different trend after the index registration of the primary case subject. We used a three-level linear probability model with individual drug abuse registrations (yes or no) during the six specified time periods nested within each secondary case and each control case that in turn were nested within a stratum consisting of both individuals at risk of becoming secondary case subjects and their matched counterparts. This model takes into account the repeated measures for each secondary case subject and each control subject. In the model, we included a dummy variable defining case or control and dummy variables for the different time periods, except within 2–3 years before drug abuse registration of the index case subject who was used as a reference. Finally, we included interaction terms between the five time dummy variables on the one hand and the case or control covariate on the other. A significant interaction term suggests that the slope of drug abuse was steeper among possible secondary case subjects than among their matched controls during the specific time period. The use of this linear probability model, from which our result is the percent gain in prevalence among at-risk secondary case subjects after index registration of the primary case subject, is warranted as an alternative logit model—which could produce odds ratios—would not give us the ability to explore the common trend assumption (
23).
We replicated the analyses using different definitions of geographical proximity. First, we used Small Area Market Surveys (SAMS), a definition of neighborhoods used by Statistics Sweden, the Swedish government-owned statistics bureau. There are approximately 9,200 SAMS throughout Sweden, with an average population of 1,000. Second, we used municipalities. There are 290 municipalities in Sweden, and they comprise the country’s lower-level local government entities. Pairs included in the household analyses were not included in the SAMS analyses, and pairs included in the household or SAMS were not included in the municipality analyses. Thereafter, we replicated the same approach using full-sibling pairs and first-cousin pairs, and thus the primary case subject was an individual who was registered for drug abuse at a time when his or her sibling or cousin was between 19 and 23 years old and resided in the same household, SAMS, or municipality as the primary case subject.
In all analyses, we investigated within- and across-sex transmission. For the parent-offspring analyses, we stratified the analyses on the basis of age difference: <20 years, 20–30 years, and >30 years. For siblings and cousins, we used 1–3 years, 4–6 years, and 7–10 years. For siblings and cousins, we also stratified on the basis of whether the primary case subject was 1–3 years older or 1–3 years younger. In the stratified analyses, we combined the household and SAMS samples to increase power.
All statistical analyses were performed with SAS, version 9.4, and the R package lme4 (
24).
Discussion
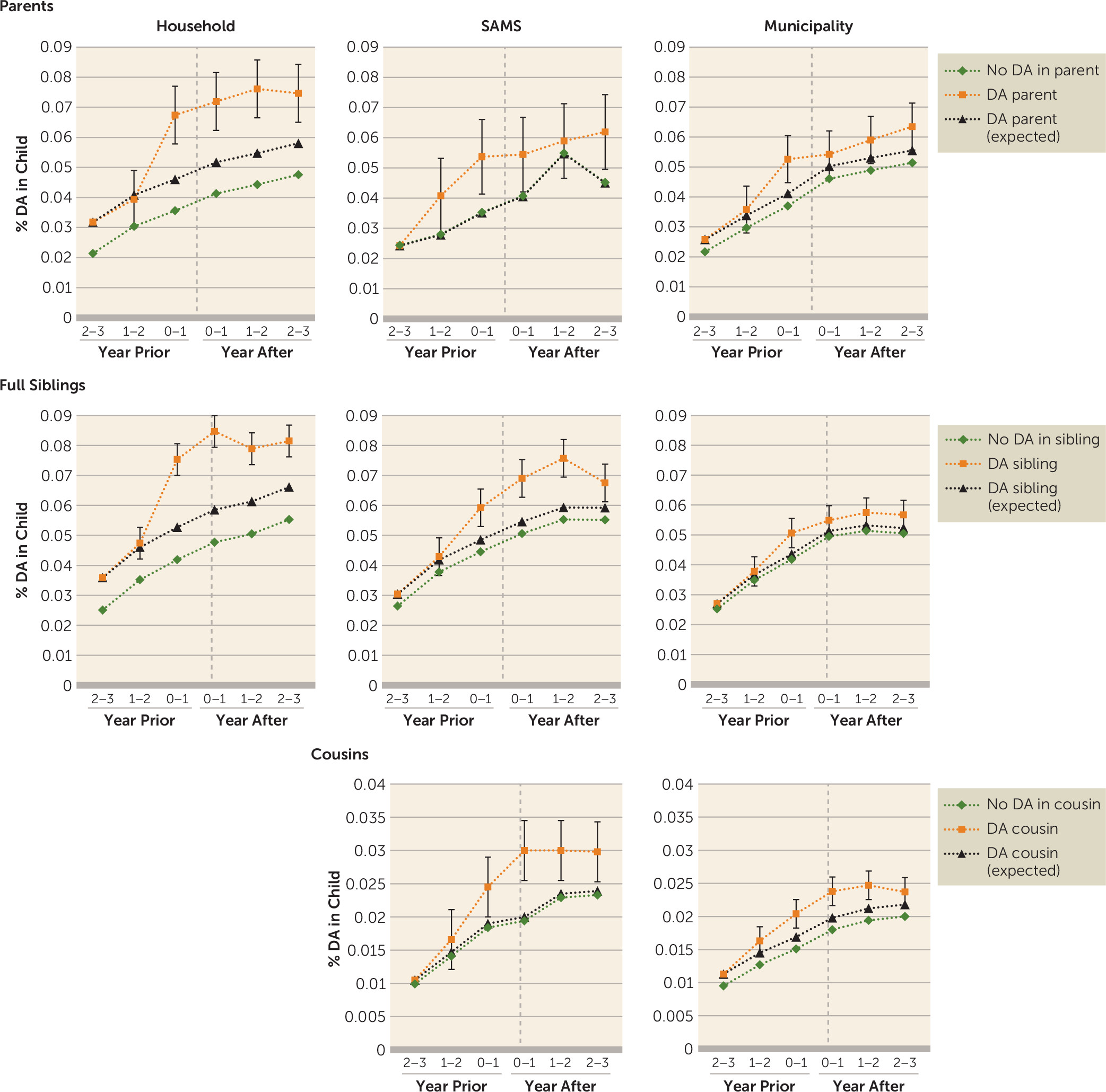
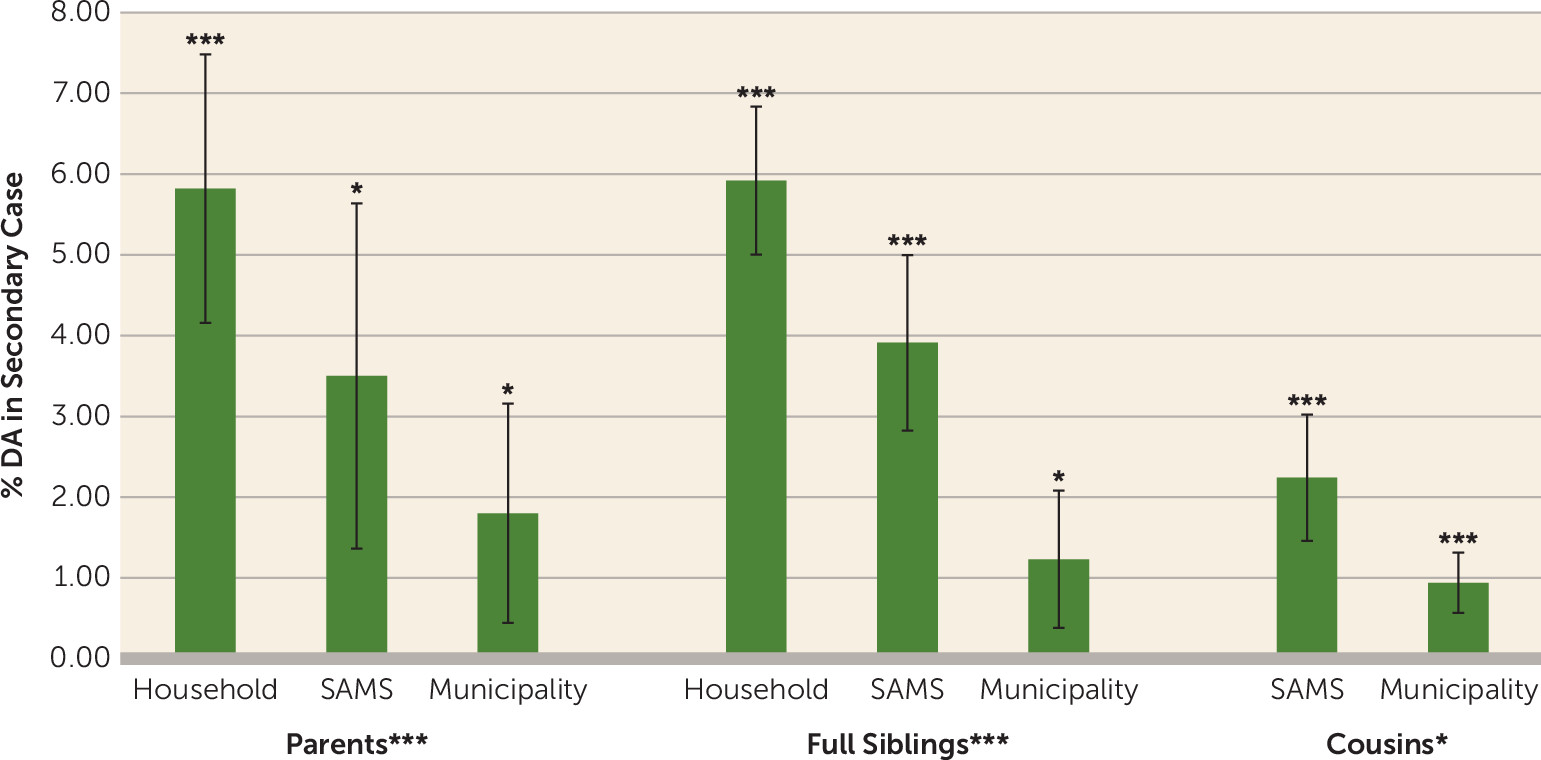
We examined evidence for a contagion model of environmental transmission of drug abuse within families. We used the date of drug abuse registration of primary case subjects to define periods of high risk among their relatives. Compared with matched control subjects, an appreciably increased risk for drug abuse was found among relatives 1–3 years after the index drug abuse registration of the primary case.
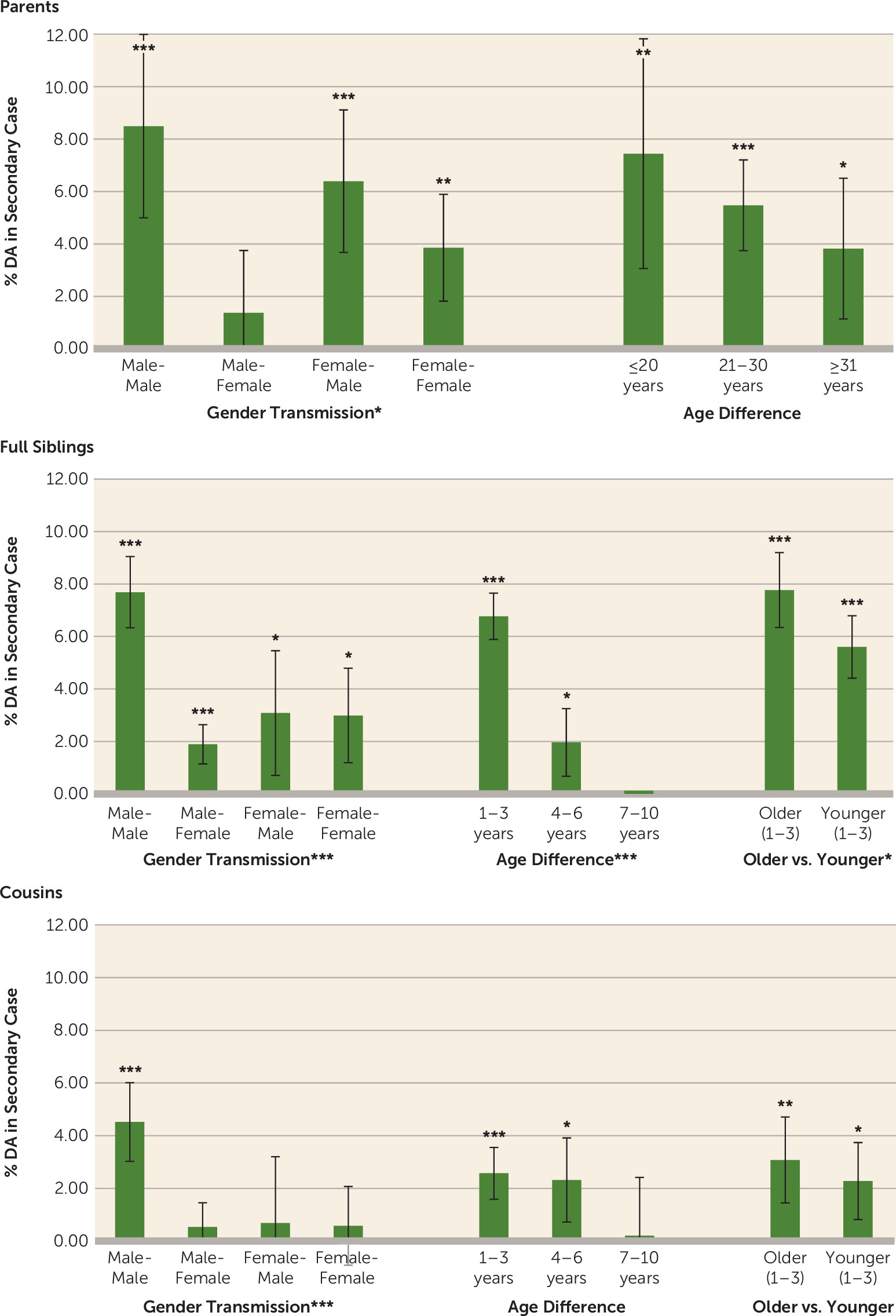
The validity of our results is supported by six findings. First, within parent-offspring, sibling-sibling, and cousin-cousin pairs, transmitted risk was strongly correlated with geographical proximity. Second, when proximity was controlled for, transmitted risk was related to familial closeness, with stronger transmission from parents to offspring and sibling to sibling than from cousin to cousin. Third, within types of relatives, transmitted risk was stronger in those closer in age. Fourth, consistent with research showing that older siblings have greater influence on younger siblings compared with the reverse (
25–
29), we observed the same effects for drug abuse transmission. Fifth, strength of transmission strongly depended on the sex composition of the pair and was consistently highest for male-male pairs and second highest for female-male pairs. This is congruent with research showing that the decision to use psychoactive substances is more affected by peer influences and the desire to conform to subgroup values among males compared with females (
30,
31). Among Swedish high school students, peer deviance was a stronger predictor of drug use among males compared with females (
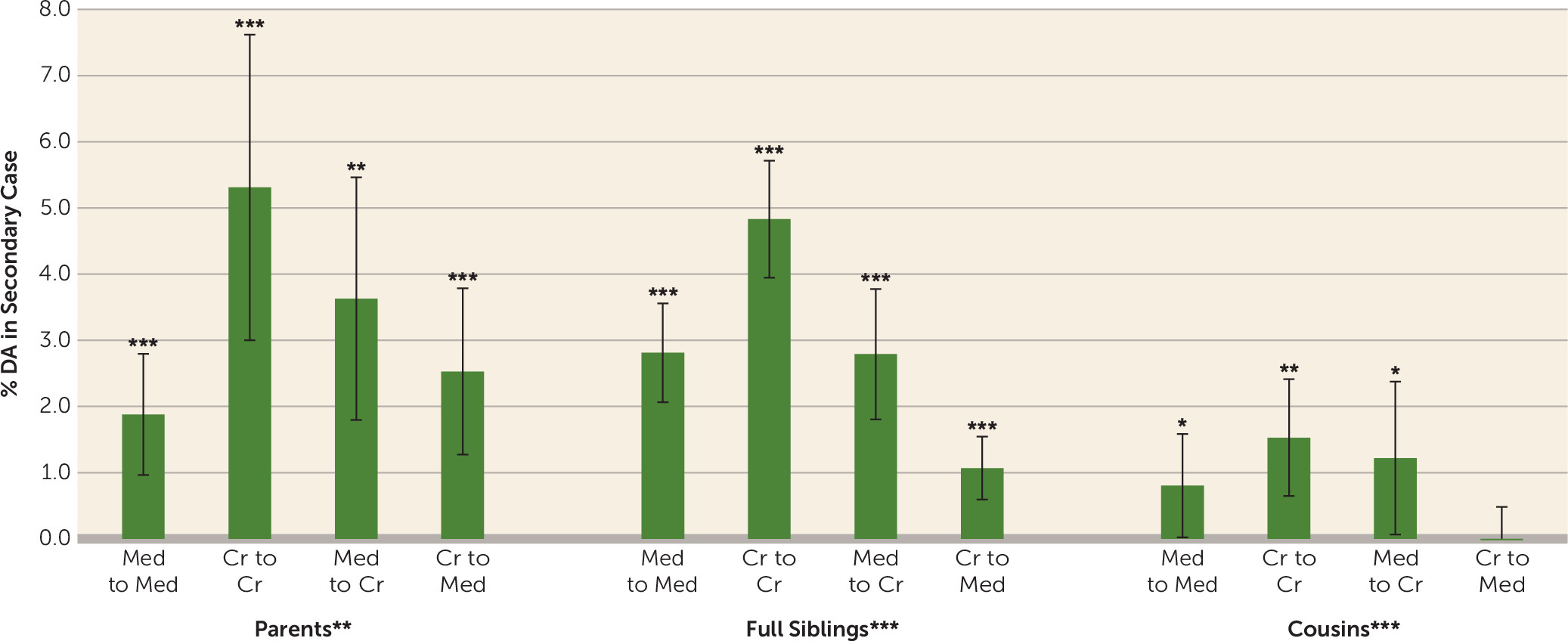
32). Sixth, in the two most common substance abuse categories (opiates and cannabis), greater within-substance transmission than between-substance transmission was observed in siblings, which suggests direct transmission, although such resemblance could arise from local patterns of drug availability.
Although we examined only biological relatives, our findings are similar to those we reported previously on marital pairs in which neither spouse had prior drug abuse registrations (
33). When one spouse had a first drug abuse registration, the risk for drug abuse registration for the other spouse increased substantially in the subsequent year and remained elevated over the next 4 years (
33).
In considering the underlying basis for the transmission of drug abuse within relatives, we suggest the utility of differentiating two mechanisms. The first, a form of social learning (
34), would consist of the primary case subject transmitting to relatives both positive attitudes toward drug use and a positive model for the drug use itself. Much of our results can be explained by a key postulate of social learning theory: the potency of a role model depends on the strength of the identification with the individual. We would predict that a male teenager would, on average, identify more strongly with a young father and an older brother close in age than with a mother or a younger cousin. Our results demonstrate this pattern. The second mechanism for environmental transmission of drug abuse is simpler: the primary case subject either supplies the substance to the at-risk relatives or tells them specifically how to obtain the substance. That this process is also at work is evidenced by the much stronger within-substance transmission than cross-substance transmission of drug abuse between relatives.
The drug abuse field is legitimately excited by advances in molecular techniques that will further elucidate the underlying nature of the genetic contributions to drug abuse. However, given that our case and control subjects were matched for genetic and familial risk, a model in which familial aggregation of drug abuse is solely a result of genetic factors could not plausibly explain the observed substantial temporal relationship of onsets in pairs of relatives.
Using triangulation—the application of multiple complementary methods to the same research questions (
35)—we have demonstrated, in traditional twin, sibling, and adoption studies (
2,
36), in novel designs using stepparents (
37) and marital partners (
33), in age differences within sibling (
33) and cousin pairs (
38), and now in contagion models, that environmental factors also contribute substantially to the transmission of drug abuse within families. Importantly, these environmentally informative designs highlight potential foci for prevention, including the identification of individuals at particularly high risk, such as close relatives of an individual with recent-onset drug abuse.
Limitations
Our findings should be interpreted in the context of six potential methodological limitations. First, drug abuse registration is not a precise index of the onset of drug abuse. Indeed, in most analyses, transmission of drug abuse from primary to secondary cases begins in the year before index registration, as would be expected because problematic drug use often precedes drug abuse registration by months or years. Prospective studies suggest that about 50% of individuals who transition from the use of common illicit drugs to abuse or dependence do so within 2–4 years, with faster transitions to abuse (
39–
41). Second, given the increased risk for drug abuse among secondary case subjects before index registration of the primary case, we cannot rule out reverse causation (from the secondary to primary case). To investigate this, we examined siblings residing in the same household or community in our usual way and then repeated the analyses censoring all secondary case or control subjects with a drug abuse registration in the 3 years before index registration of the primary case subject. Although rates of drug abuse were lower in the censored subsamples, case-control differences in risk were unchanged (for further details, see Figure S2 in the
online supplement), suggesting that reverse causation contributed minimally to our findings. Third, our drug abuse assessments differ qualitatively from those obtained by personal interviews and have the advantage of being objective and are unaffected by cooperation or recall bias. However, false negative reports are likely, because some drug abusers will avoid detection by registries. It is possible that by examining more severe cases of drug abuse, we overestimated the familial-environmental transmission of drug abuse. Fourth, we did not match index case and control subjects on geographical region. Could the correlations in risk between primary and secondary case subjects result from local drug abuse epidemics? To evaluate this, we compared estimates of excess rates of secondary cases among the siblings of index cases with those obtained by matching on municipality (since matching on SAMS proved to be infeasible). These estimates were 5.05% (95% CI=4.30–5.80) and 4.42% (95% CI=3.67–5.17), respectively, suggesting a modest reduction in rates of transmitted drug abuse when controlling for local period effects. Fifth, our main analyses defined the proximity of pairs of relatives (household, SAMS, or municipality) only at the time of index case registration. To determine the possible effect of this definition, we reanalyzed our sample including only pairs with maintained proximity over the entire 3-year follow-up period (for further details, see Tables S1 and S2 in the
online supplement). In this censored sample, the excess risk for drug abuse in case subjects compared with control subjects increased substantially for pairs residing in the same household and modestly for those living in the same SAMS but decreased slightly for those residing in the same municipality. Finally, contagious transmission of drug abuse does not replicate all the features of infectious disease models (
42). Future work will need to determine how instructive such models can be in understanding person-to-person transmission of drug abuse.