The wide availability and use of heroin and prescription opioid analgesics in the United States during the past decade have resulted in an unprecedented epidemic, with other countries at risk of following this trend as opioid use grows worldwide. The crisis has led to more than 300,000 opioid-related deaths in the United States during the past decade (
1), has contributed to more than 4 million years of life lost globally (
2), and since 2007 has contributed to an increase in excess of 3,000% in medical services needed for patients with opioid use or misuse, which has led to a substantial economic burden (
3). The opioid crisis has also increased awareness about the challenges that exist in treating opioid use disorder because current medications are predominantly mu opioid agonist substitution pharmacotherapies, such as methadone and buprenorphine (
4,
5). Such pharmacotherapies are associated with marked stigma and tight governmental regulation because of their potential addictive liability and diversion to the black market, further burdening clinical care and access. Thus, these medications are underutilized in the treatment of millions of people diagnosed with opioid use disorder (
6,
7).
This treatment gap for the vast number of patients with opioid use disorder highlights the urgent need to develop novel therapeutic strategies that do not target the mu opioid receptor. To address this critical need, we initiated studies (
8,
9) of cannabidiol (CBD), a nonintoxicating cannabinoid (
10,
11), as a potential treatment of opioid use disorder. Our preclinical studies (
10) demonstrated that CBD reduces reinstatement of heroin-seeking behavior specifically triggered by a prior drug-associated cue in animals with a history of heroin self-administration. The specific effects of CBD on cue-induced drug-seeking behavior are particularly important in the development of addiction therapeutics because environmental cues are one of the strongest triggers for craving, which is a core component of opioid use disorder as defined in DSM-5 (
12) and which contributes to relapse (
13,
14). Another aspect of our preclinical findings that is important for a potential medication for opioid use disorder is that the reduced heroin-seeking behavior is maintained for weeks following CBD administration (
15). Other animal studies have reported consistent findings that CBD reduces contextual drug-related memories associated with drug-seeking behavior for different substances of abuse (
11,
16).
To determine whether this preclinical evidence could be translated to humans, we conducted a series of clinical studies and demonstrated that CBD was safe in humans and did not result in adverse consequences when coexposed with a potent opioid agonist (
8), in line with its safety and tolerability even at high doses (
17). The aim of the present study was to use a double-blind, randomized, placebo-controlled design to explore the effects of acute and short-term CBD administration on craving and anxiety in heroin-addicted individuals. This was examined with the presentation of drug-associated environmental cues to induce craving and stress responsivity in these individuals because such stimuli are strong triggers for opioid use (
13,
18,
19). In addition, CBD has been shown to reduce anxiety (
20,
21), which we expected to be enhanced by the presentation of drug cues (
22). The recent finding that CBD reduces the attentional bias to cigarette cues in tobacco smokers (
23) also suggests that a potential strength of CBD for addiction treatment could be through the attenuation of the salience of drug cues. The secondary outcomes investigated in this study were general affect, cognition, and physiological factors, all of which are important in the development of medications for use in the treatment of substance use disorders. We assessed the effects of CBD administered at doses of 400 mg and 800 mg. These doses were selected on the basis of our previous human safety study with CBD (
8) and our cue-induced heroin-seeking animal model assessing CBD (
15). Moreover, this dose range overlapped known effects of CBD on biological systems relevant to craving and stress responsivity (for example, cortisol levels) (
24) and altered cerebral blood flow in limbic brain regions, such as the amygdala (
25). We hypothesized that CBD would reduce cue-induced craving and anxiety in heroin-abstinent individuals with heroin use disorder and have minimal adverse effects.
Methods
Study Design
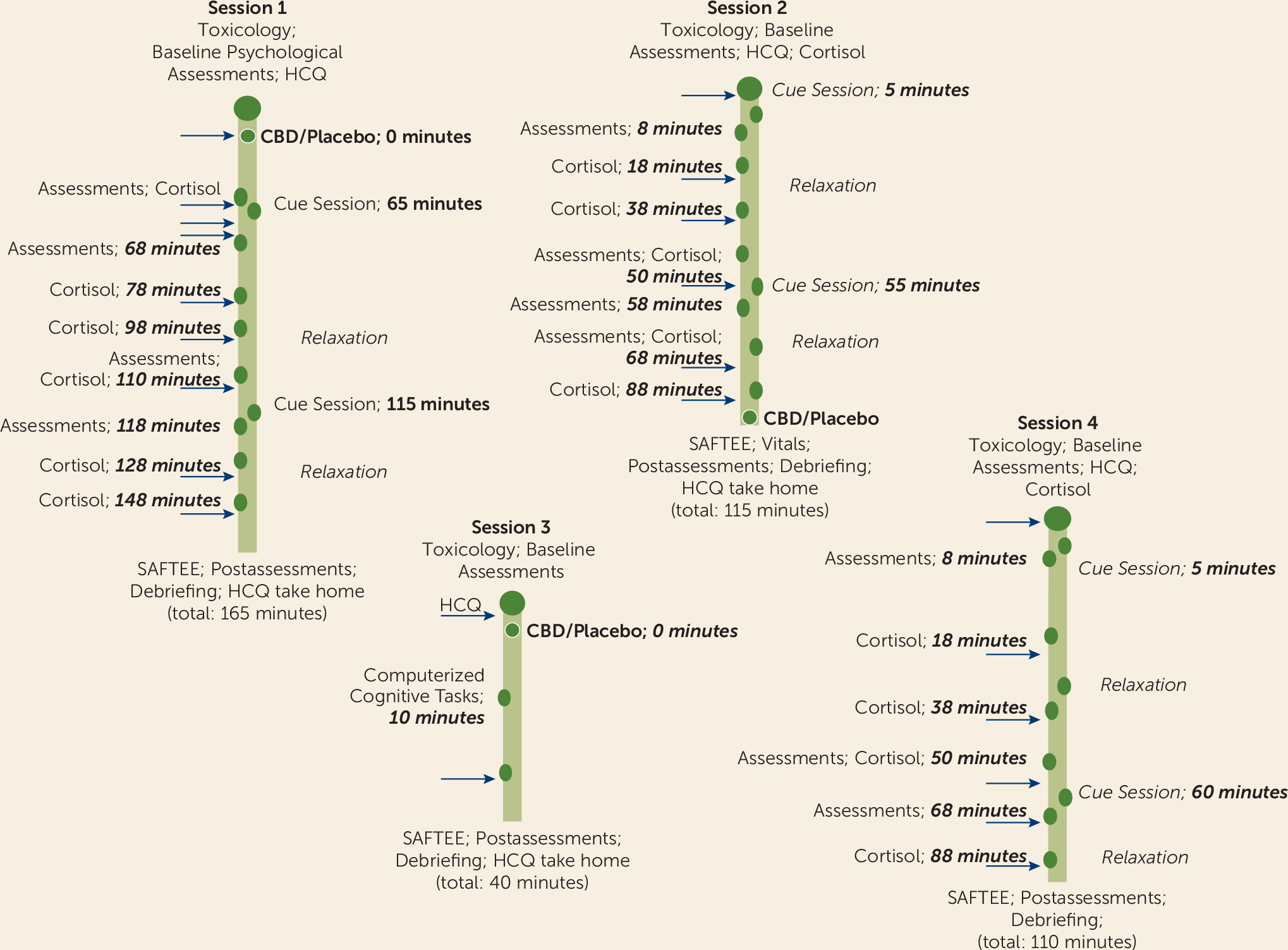
This double-blind placebo-controlled randomized clinical trial was conducted at the Mount Sinai Beth Israel Hospital in New York. The study was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai and Mount Sinai Beth Israel and conducted according to the Declaration of Helsinki and the International Conference on Harmonization’s Tripartite Guideline on Good Clinical Practice. Enrolled participants were randomly assigned to one of three treatment groups and completed four test sessions over the course of 2 weeks (see Figure S1 in the online supplement). Three sessions occurred on consecutive days in which the test drug was administered daily, and the final session occurred 1 week after the last CBD or placebo administration.
Participant Recruitment and Treatment Assignment
Abstinent men and women with heroin use disorder who were between 21 and 65 years of age were recruited through institutional review board–approved advertisements posted in local newspapers and at social service organizations, halfway houses, and college campuses in New York City. Participants were also directly recruited from Mount Sinai Health System clinical addiction treatment sites. Potential participants were assigned a unique identification number and screened in a brief telephone interview. Those who met preliminary criteria were scheduled for a prescreening visit at Mount Sinai Beth Israel, where written informed consent was obtained after participants received a complete description of the study, and a comprehensive screening was performed to assess individuals’ general medical and mental health. A basic physical examination, urine toxicology test (Medimpex United, Bensalem, Pa.), breathalyzer, clinical laboratory blood testing (complete blood count, liver function, and chemistry panel), and electrocardiography were conducted to evaluate medical eligibility for participation. The Structured Clinical Interview for DSM-IV was used to diagnose drug use and dependence. The Mini International Neuropsychiatric Interview was used to detect axis I psychiatric conditions as defined in DSM-IV, and the Clinical Opiate Withdrawal Scale was administered to evaluate opiate withdrawal. Healthy participants who met DSM-IV criteria for opioid dependence were enrolled in the study. Participants were excluded if they tested positive for any psychoactive drug other than nicotine; met DSM-IV criteria for any axis I diagnosis other than heroin or nicotine dependence within the previous 3 months; were being maintained on methadone, buprenorphine, or an opioid antagonist; had a significant medical history or condition; had hypersensitivity to cannabinoids; or showed signs of acute heroin withdrawal.
Patients were randomly assigned to receive 400 mg of CBD, 800 mg of CBD, or matching placebo (excipients alone). The randomization schedule was produced by an investigator independent from the study, held centrally, and not divulged to anyone involved in the trial. A stratified block randomization schedule was used for equal assignment to treatment groups, with a set of permuted blocks generated for each sex to reduce imbalances among groups. A unique treatment number was used to identify each carton of investigational medicinal product and its contents; CBD and placebo containers were identical in appearance. After randomization, patients were allocated an investigational medicinal product pack in sequential treatment number order.
Test Drug
The CBD oral solution (100 mg/mL; Epidiolex) and the matched placebo solution were obtained from GW Pharmaceuticals (Salisbury, Wiltshire, United Kingdom). The oral CBD solution was administered at either 400 mg or 800 mg. Investigational CBD was also made up of ethanol (79.0 mg/mL), sucralose (0.5 mg/mL), strawberry flavor (0.2 mg/mL), and refined sesame oil (to a volume of 1 mL). The placebo oral solution was identical in appearance, taste, and composition except for the active ingredient of pure CBD. Oral solutions of 400 mg and 800 mg of CBD were approximately 5 mg/kg and 10 mg/kg of participant weight, respectively (
Table 1). CBD rapidly appears in plasma, reaches peak plasma concentration in 3–4 hours (
8), and has a half-life of 18–32 hours (
17). CBD or placebo was administered once daily for 3 consecutive days starting on the first test session day, session 1.
Test Sessions
The experimental design conducted in each test session is presented in
Figure 1. All participants were screened for drug use and alcohol intoxication before each test session, and women were additionally screened for pregnancy. The Heroin Craving Questionnaire and visual analogue scale for anxiety (VAS-A) were administered prior to the start of all test sessions. The Clinical Opiate Withdrawal Scale was administered at screening and during session 4 (which was conducted 7 days after participants had been monitored in the laboratory) to identify any signs of opioid withdrawal. To avoid any potential coexposure confounding effect, participants who tested positive for drugs (except for cannabinoids) or showed clinical signs of intoxication were withdrawn from the study prior to the start of any session in which the test drug was being administered.
Measures of opioid craving (assessed using the visual analogue scale for craving [VAS-C]), anxiety (assessed using the VAS-A), positive and negative affect (assessed using the Positive and Negative Affect Schedule [PANAS]), vital signs (skin temperature, blood pressure, heart rate, respiratory rate, and oxygen saturation; automated measures obtained by machine), and salivary cortisol levels were obtained at different times during the sessions. Detailed information on each of the measures is provided in Table S1 in the online supplement.
Cue sessions.
Participants were exposed to neutral and drug-related cues at two points during the course of test sessions 1 (acute CBD or placebo administration), 2 (24 hours after CBD or placebo administration), and 4 (7 days after the third and final daily CBD or placebo administration). Cues were given at the same time of day in each test session. The 3-minute neutral cue condition consisted of a video showing relaxing scenarios, such as scenes in nature. The drug cue condition was a 3-minute video that showed intravenous or intranasal drug use, depending on the participant’s reported preferred route of drug use. Immediately after the presentation of the cue stimuli during test session 2, participants were also exposed to neutral objects or to heroin-related paraphernalia (e.g., syringe, rubber tie, and packets of powder resembling heroin) for 2 minutes. The order in which the neutral and drug cues were presented was counterbalanced and randomized across participants.
Cognitive test session 3.
The aim of session 3 was to assess the protracted effects of the prior short-term CBD exposure on general cognition, for which baseline measures had been obtained in the prescreening session. Approximately 10 minutes after CBD or placebo administration, when no immediate effects of the drug would be expected, participants completed computerized versions of the Digit Symbol Substitution Task, the Digit Span Test–Backward, and the Continuous Performance Test; vital signs were obtained; and the VAS-C and VAS-A were administered. No cues were presented in session 3.
CBD or placebo sessions.
Session 1 assessed the acute effects of CBD or placebo. Either CBD or placebo was administered 60 minutes before the first cue test, when significant plasma CBD concentrations were detectable (60 μg/L and 80 μg/L for 400 mg and 800 mg, respectively) (
8) and when effects on cue-induced craving and anxiety were observed in a pilot study (
9). Session 2 assessed the protracted effects 24 hours after the first CBD or placebo administration; the second dose of CBD or placebo was administered at the end of the test session, after the cue tests had been finished. Session 3 assessed the effects of the prior short-term accumulated CBD exposure on cognition; the third (final) dose of CBD or placebo was administered just prior to starting the cognitive tasks.
The study data were managed using the Electronic Research Application Portal data capture tool housed on a secure institutional server.
Discharge.
Before participants were discharged from each session, they were debriefed, and their vital signs and well-being were assessed by the clinical staff. A standardized safety and adverse events questionnaire (Systematic Assessment for Treatment Emergent Events) was used to assess any adverse events or off-target effects. To attenuate any craving or anxiety that may have arisen during test visits, participants were guided through a series of muscle-tension relaxation exercise techniques via an audio recording. After the relaxation exercises, the VAS-C and VAS-A were readministered to assess craving and anxiety levels prior to discharge. If there was a ≥3-point difference between these scores and the precue VAS-C and VAS-A scores, participants would be referred for further clinical evaluation and assistance; however, no participant required that service during this study. Participants were reimbursed for their participation after each visit and given the Heroin Craving Questionnaire to take at home after sessions 1, 2, and 3. At the end of the final session, participants took part in an exit interview that assessed general experience in the study and ensured that participants were offered appropriate resources to seek treatment.
Data Analysis
Given that this study used a repeated design across sessions for some of the outcomes, a linear mixed-model repeated-measures analysis using the SAS procedure MIXED (SAS Institute, Cary, N.C.) was employed to assess the changes in cue-induced in-clinic VAS-C, VAS-A, and PANAS scores. Changes in the Heroin Craving Questionnaire out-of-clinic craving scale score were assessed using a repeated-measures analysis conducted with the MIXED procedure. At the first stage of all analyses, the distributional characteristics of all continuous explanatory and outcome variables were assessed using skewness and kurtosis indicators. If necessary, transformations were used to normalize a variable. To determine the effectiveness of the randomization procedure for assignment to the drug group, one-way analyses of variance were conducted on the session 1 baseline values of each of the outcome variables as a function of drug group. Nonsignificant drug group baseline differences were taken as indicators of effective randomization. Because a crossover design assumes that there are no carryover effects from one assessment period to the next, this possibility was tested using procedures in the Number Cruncher Statistical System User’s Guide II. Once the analyses indicated that carryover effects were absent, the main analyses of the crossover design variables were undertaken.
Difference scores between the precue baseline scores and postcue scores within each session were calculated and used as outcomes in the analytic models. The models themselves were developed sequentially in which time was entered first (because there were multiple sessions and two events within each session for the crossover outcomes; that is, neutral and drug cues). This procedure was followed by entering the variable describing the sequence in which the drug and neutral cues were given, the drug group variable, and the cue indicator (neutral or drug cue). A single first-order interaction term (drug group by cue) was entered last because it was hypothesized that the cues would be responded to differentially depending on the drug group to which the research participant was assigned. Any significant mean differences for the main effect analyses or interaction terms were followed up by Tukey-adjusted post hoc mean comparisons.
Results
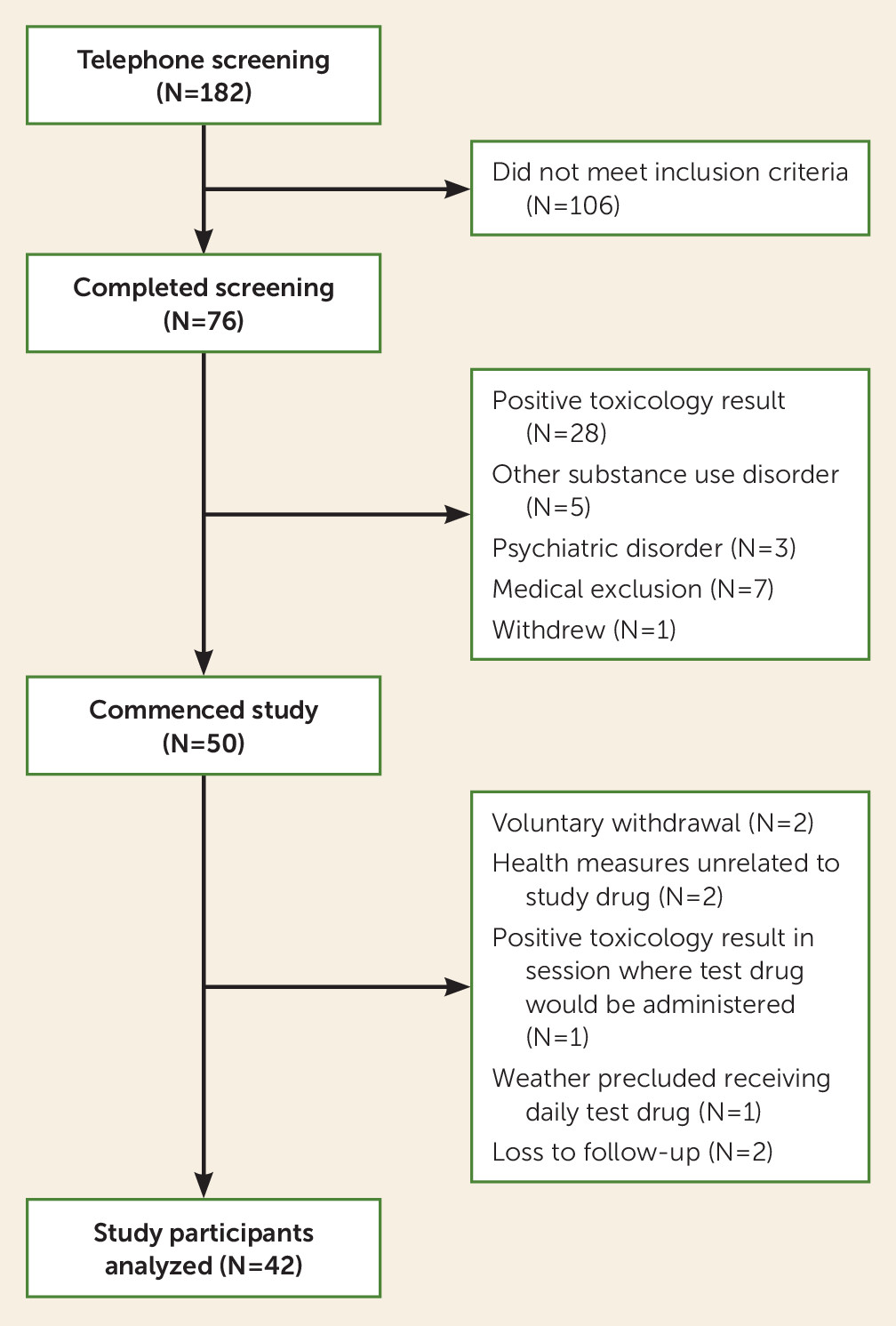
Of 50 participants who began the study, eight were excluded (
Figure 2) because of voluntary withdrawal, positive toxicology results prior to session 4, health issues unrelated to the study drug or placebo, weather preventing daily test drug administration, or loss to follow-up. The data presented here are from the remaining 42 participants (
Table 1). Most participants (78.6%) indicated preference for intranasal heroin use, 83.3% reported currently using more than 10 bags of heroin (one bag=1 g) daily, and on average, participants had been using heroin for approximately 13.2 years. The majority of participants (64.3%) had been abstinent from heroin use for less than 1 month, 14.3% for 1–2 months, and 21.4% for 2–3 months. In addition to heroin use disorder, most participants had a history of alcohol use disorder or cannabis use disorder but were not currently diagnosed with those disorders. The majority were also tobacco smokers. A small percentage (11%) of participants reported a history of major depressive disorder or bipolar disorder. Seventy-one percent of participants had hypertension, 25.3% were HIV positive, and 17.8% had hepatitis C. Drug history, psychiatric history, and medical history did not differ significantly among the three groups.
Initial analyses indicated that, with the exception of two cognitive test variables (described below), all the continuous variables were normally distributed and did not require transformation. There were no baseline differences on any of the outcomes of session 1 as a function of drug group assignment, indicating that the randomization was effective. The results of all t tests examining potential carryover effects between sessions were nonsignificant, indicating that carryover effects were unlikely. Although our sample included few women (N=7), sex was either significant or approached significance in many of the tests and thus was considered a covariate in all analyses.
Craving
Cue-induced craving (VAS-C).
There was no significant difference between the groups in their baseline craving scores. There was a significant contribution of sex (F=4.05, df=1, 78, p=0.0476), with women reporting nearly twofold greater craving than men. The overall analysis across all sessions showed a significant difference in the cue condition (F=34.55, df=1, 82, p<0.0001). Craving scores for all participants after experiencing the drug cues (adjusted for their baseline craving score) were significantly higher (mean difference score=1.09) than when they were exposed to the neutral cues (mean difference score=−0.02). There was also a significant main effect of drug group (F=5.74, df=2, 78, p=0.0047). Across all sessions, individuals receiving placebo reported significantly greater craving after the drug cues (mean difference score=0.93) compared with participants in either of the CBD groups (mean difference score for 800 mg of CBD=0.23; mean difference score for 400 mg of CBD=0.44). There was no significant difference in craving scores between the groups of participants administered the two CBD doses, indicating that both doses equally reduced craving.
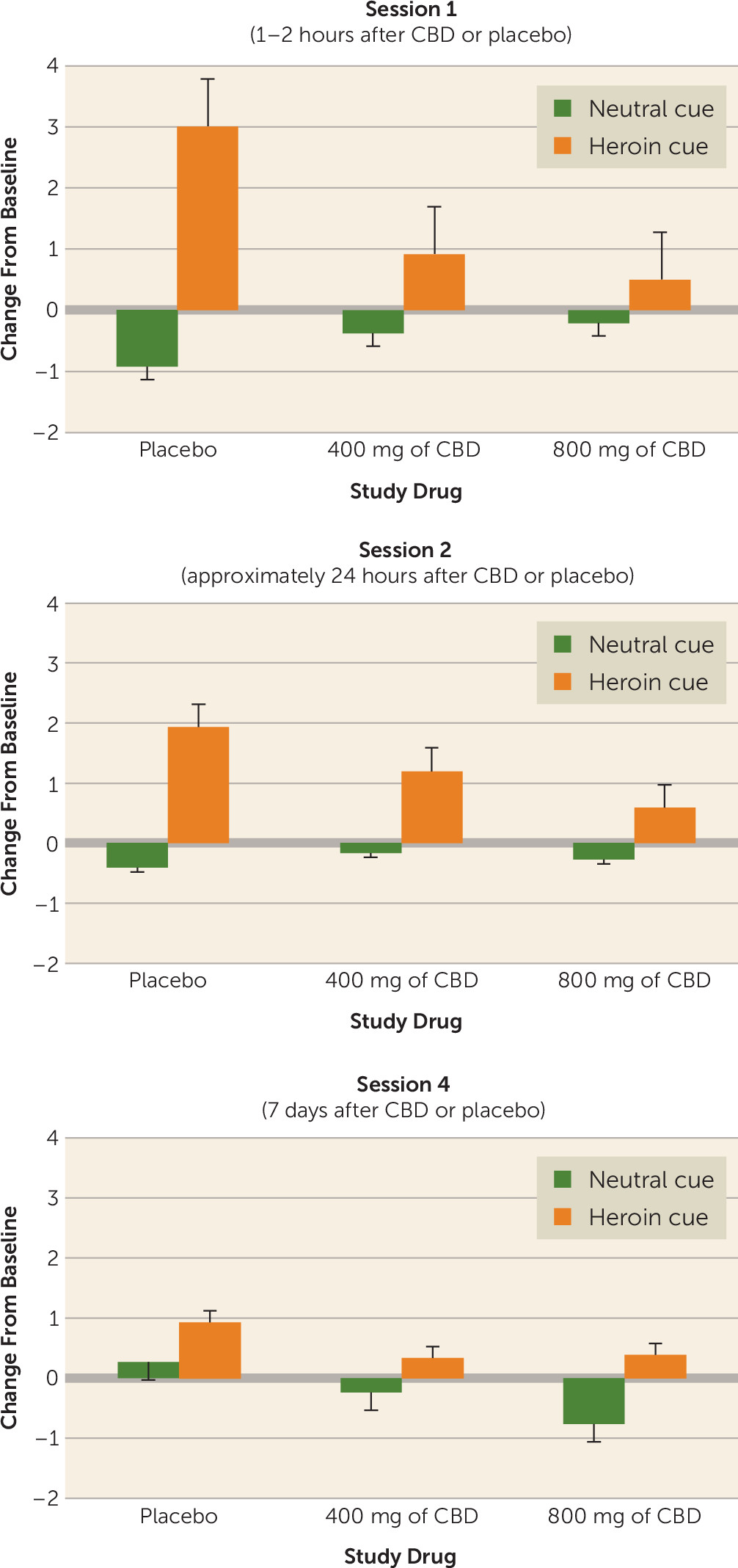
The VAS-C scores for each session are summarized in
Figure 3. The effects of CBD on craving were most prominent during session 1, which was conducted from 1 to 2 hours after CBD administration. There was a significant interaction between the cue condition and drug group (F=5.15, df=2, 38, p=0.0105). Consistent with the literature (
19,
26) and clinical expectation, when participants were exposed to the drug cue, craving scores were significantly increased (mean difference score=1.47). By contrast, craving scores remained unchanged when the participants were exposed to the neutral cue (mean difference score=−0.51). The highest level of craving was evident for participants receiving placebo under the drug cue condition, followed by those who received 400 mg of CBD and then those who received 800 mg of CBD. Lower craving scores 24 hours after administration of the first CBD dose did not reach a level of significance. There was an apparent habituation of the cue-induced craving in participants receiving placebo in session 2, with a marked drop (approximately 40%) in craving scores. By contrast, the craving scores in session 2 for participants in both CBD groups remained the same as those observed in session 1. In addition, although the apparent habituation to the drug cues (a twofold reduction in cue-induced craving) for participants receiving placebo continued into session 4, participants in the CBD groups remained relatively stable in their low cue response across sessions. Nevertheless, in session 4, which occurred 1 week after the last CBD administration, a significant main effect of group was evident (F=4.58, df=2, 37, p=0.0167). Participants who had received placebo in the previous week reported significantly greater craving (mean difference score=0.94) compared with those who had received 800 mg of CBD (mean difference score=0.39). There was no significant difference in craving scores between participants in either CBD group, and craving scores in the group who received 400 mg of CBD did not significantly differ from those in the placebo group.
Out-of-clinic Heroin Craving Questionnaire.
General craving was also assessed outside of the laboratory cue session using the abbreviated Heroin Craving Questionnaire that participants took home to complete (results are available in Table S2 in the online supplement). There were no significant group differences in craving reported at baseline and no significant sex difference in craving as measured with this scale. The only statistically significant factor was time (F=2.80, df=6, 44, p=0.0213), with craving strongest at baseline (mean=59.42) and decreasing significantly at each subsequent time point examined.
Anxiety
Cue-induced anxiety (VAS-A).
Sex was not significantly associated with anxiety in any session (F=3.27, df=1, 78, p=0.0745), but female participants tended to report greater anxiety (mean difference score=0.82) than male participants (mean difference score=0.31). The main effect of cue condition was significant (F=53.30, df=1, 80, p<0.0001). Similar to the VAS-C condition, the drug cues were significantly associated with greater baseline-adjusted anxiety (mean difference score=1.28) than that experienced with the neutral cues (mean difference score=0.16). The main effect of drug group was also significant (F=5.15, df=2, 78, p=0.0079). Across all sessions, participants in the placebo group reported significantly greater baseline-adjusted anxiety after the cues (mean difference=0.97) compared with those in both the 400 mg (mean difference=0.48) and 800 mg (mean difference=0.24) CBD groups. However, there was no significant difference in anxiety between the two CBD groups. The interaction between cue condition and drug group was also significant (F=3.94, df=2, 80, p=0.0233). For all groups, the level of anxiety was greater when participants were exposed to the drug cues than when they were exposed to the neutral cues. However, this difference was most pronounced for those receiving placebo, followed by those who received 400 mg of CBD and then those who received 800 mg of CBD.
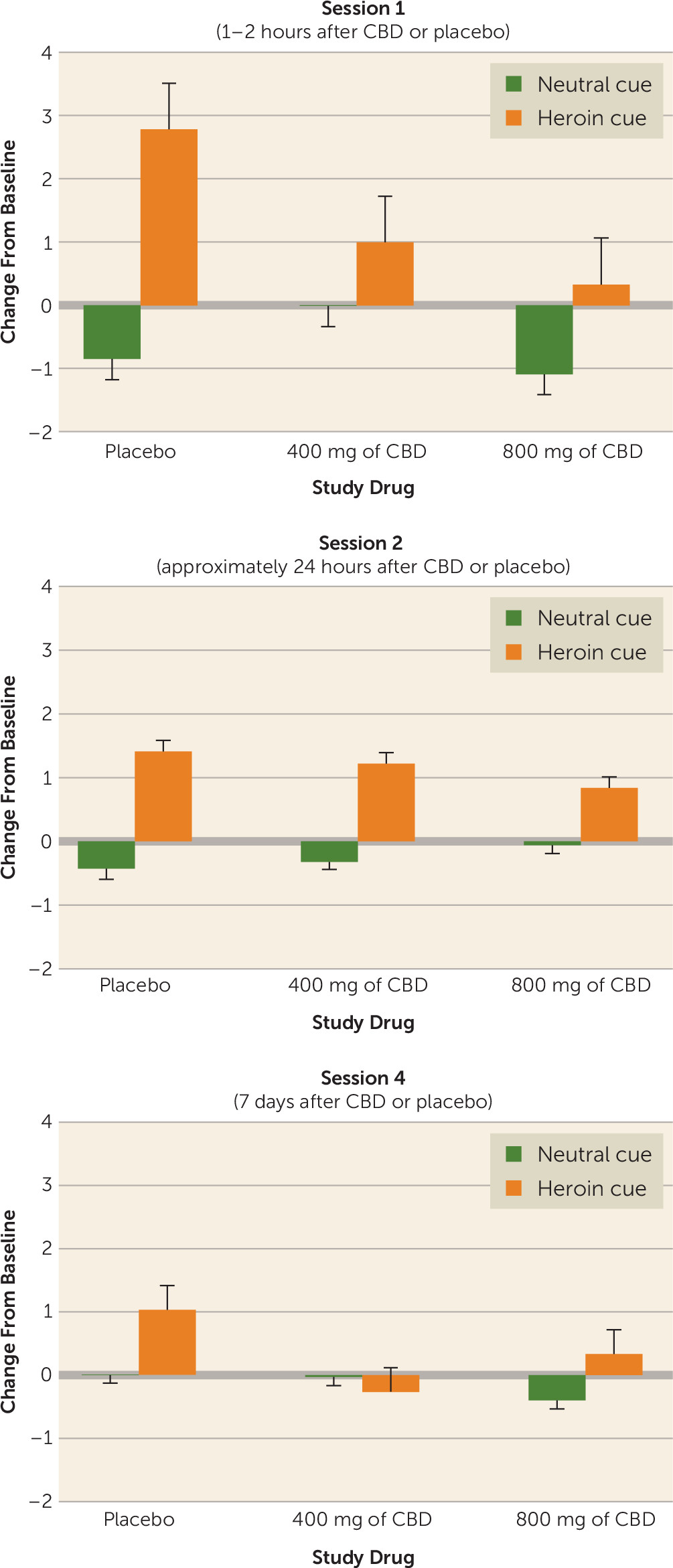
In session 1, women reported a significantly greater (F=4.82, df=1, 37, p=0.0344) baseline-adjusted increase (mean=0.89) in anxiety compared with men (mean=−0.18). The cue condition was also significant (F=29.22, df=1, 38, p<0.0001). Participants exposed to the drug cue had significantly higher anxiety scores (mean difference score=1.37) than when they were exposed to the neutral cue (mean difference score=−0.65) (
Figure 4). The interaction between the cue condition and drug group was also significant (F=3.98, df=2, 38, p=0.0270). After experiencing the drug cue, there was a significant increase in anxiety (mean difference score=2.78) for participants in the placebo group, followed by participants who received 400 mg of CBD (mean difference score=0.99) and then those who received 800 mg of CBD (mean difference score=0.33). For the neutral cue, the mean difference score for all three drug groups indicated decreased anxiety after viewing the cue. A similar pattern was evident in session 2, where anxiety scores were significantly higher after experiencing the drug cues (mean difference score=1.16) than after exposure to the neutral cue (mean difference score=−0.07), although there was not an overall significant drug group difference. In session 4, the main effect of drug group was significant (F=3.64, df=2, 36, p=0.0363), with participants receiving placebo reporting significantly greater anxiety (mean difference score=0.53) than those receiving 400 mg of CBD (mean difference score=−0.14) or 800 mg of CBD (mean difference score=−0.03). There was no significant difference in anxiety between participants in the two CBD groups.
Secondary Outcomes
Positive affect scores (PANAS).
An overview of the PANAS scores is provided in Table S3 in the online supplement. There was a significant main effect of drug group in session 1 (F=4.60, df=2, 37, p=0.0165) for the positive affect scores. In this session, participants receiving 400 mg of CBD reported significantly greater baseline-adjusted positive affect driven by the drug cue response (mean difference score=2.70) compared with those receiving 800 mg of CBD (mean difference score=−2.10). The baseline-adjusted positive affect in participants in the placebo group did not differ from that in the CBD groups. No significant effect in positive affect was detected in sessions 2 or 4.
Negative affect scores (PANAS).
Cue was the only main effect that was statistically significant (F=24.54, df=1, 80, p<0.0001) in the overall analysis of the negative affect scale across all sessions. The overall level of baseline-adjusted negative affect scores was significantly higher (mean difference score=1.57) for the drug cue than for the neutral cue (mean difference score=−1.48). In session 1, the cue condition was significant (F=8.49, df=1, 38, p=0.006). When participants were exposed to the drug cue, negative affect scores were significantly higher (mean difference score=2.42) than when they were exposed to the neutral cue (mean difference score=−0.89). The main effect of drug group was also significant (F=3.42, df=2, 37, p=0.0433). Participants receiving placebo expressed the greatest increase in negative affect (mean=5.31), whereas those receiving 800 mg of CBD expressed the lowest increase in negative affect (mean=0.79) (see Table S3 in the online supplement). However, the difference in negative affect between those receiving 800 mg of CBD and those receiving placebo did not reach statistical significance (p=0.06). There were no significant differences between the treatment groups in sessions 2 or 4, although the drug cue continued to increase negative affect scores.
Cognitive Performance
Cognition was assessed at prescreening and in session 3. There were no overall group differences at the prescreening for any of the cognitive tasks (Digit Symbol Substitution Task, Digit Span Test–Backward, and Continuous Performance Task) in regard to the correct responses, suggesting that the groups were similar at baseline. There were also no significant differences in the change from baseline in the cognitive performances measured in session 3.
Physiological Measures
Heart rate.
There was no overall group difference in baseline heart rate for each session, indicating that heart rate in the groups did not change across sessions owing to the repeated administration of CBD or placebo. In session 1, there was a steep decrease in heart rate across the session, with a significant interaction between time and cue (F=2.199, df=9, 324, p=0.0218). The first cue contributed to the strongest effect, which was prolonged and thus affected the later cue. Assessing only the first cue presentation indicated that participants receiving placebo had elevated heart rate associated with the drug cue but not with the neutral cue, for which heart rate continued to decrease over time (see Figure S2 in the online supplement). The drug cue–related increase in heart rate was absent in both groups receiving CBD. When exposed to the neutral cue, participants receiving CBD had a continuous decrease in heart rate similar to that observed in the placebo group. Similarly, in session 2, heart rate tended to be increased in participants receiving placebo who were shown the drug cues, but this effect was not evident in the individuals administered CBD 24 hours before the test. By session 4, the drug cue no longer increased heart rate in any group.
Temperature.
In session 1, there was a strong tendency for an interaction of cue and group (F=1.629, df=2, 35, p=0.052), such that participants receiving placebo had elevated temperature with exposure to the drug cue but not to the neutral cue. No increase in temperature was apparent in the CBD groups. A similar effect was apparent in session 2, whereas no differences were apparent in session 4.
Blood pressure, respiration rate, and oxygen saturation.
No significant group effects were apparent for these measures in any session.
Salivary cortisol levels.
There were no significant group differences in baseline salivary cortisol levels. There was, however, a significant group difference in association with the cues, with an interaction between drug group and cue across time in session 1 (F=6.156, df=2, 70, p=0.003). There was no significant group difference for the neutral cues, whereas drug cues increased cortisol levels from the precue baseline in participants receiving placebo 15 and 35 minutes after the cue presentation (see Figure S2 in the online supplement). By contrast, the drug cues failed to increase cortisol levels in participants administered CBD at 400 mg or at 800 mg, leading to a significant difference between the placebo group and the group receiving 400 mg of CBD (p=0.049); the difference between those receiving placebo and those receiving 800 mg of CBD fell short of significance (p=0.09). There remained a modest tendency in session 4 for the drug cue, but not the neutral cue, to increase salivary cortisol levels in participants who had received placebo the previous week but not in those who had been administered either dose of CBD. This was, however, not statistically significant with sex as a covariate.
Toxicology Test
Urine toxicology tests detected positive cannabinoid or tetrahydrocannabinol (THC) levels in some participants. In the placebo group, one participant had positive THC levels in session 2 (participant acknowledged out-of-clinic cannabis use), and thus these data were excluded from analyses. Most of the participants with positive cannabinoid toxicology test results had received CBD (all of these participants denied cannabis use). For the 400 mg CBD group, five participants tested positive (three in session 2, and five in session 3), of whom three were the same individuals in both sessions. For the 800 mg group, five participants tested positive (three in session 2, and five in session 3), of whom three were the same individuals in both sessions. However, no participants had positive THC toxicology test results 1 week after the final CBD administration, in session 4. Regarding other drugs, two participants (one in the placebo group and one in the 400 mg CBD group) tested positive for opioids in session 4. One participant had positive opioid toxicology test results prior to session 4 but had received placebo; thus, this participant did not complete the study, and all data from this participant were excluded from analyses.
Adverse events.
Consistent with previous reports (
27,
28), no serious adverse events were noted in association with CBD administration throughout the duration of the trial. Mild diarrhea was reported in three participants, headache in three (two of whom had received placebo), and tiredness or fatigue was reported by two participants (one had received placebo; the other, 800 mg of CBD) (see Table S4 in the
online supplement).
Discussion
The results of this double-blind randomized placebo-controlled trial indicated that administration of 400 mg or 800 mg of CBD reduced cue-induced craving and anxiety in heroin-abstinent individuals, suggesting a potential role for CBD to alleviate clinical signs and symptoms critical to the continued cycle of addiction. The effects of CBD on drug cue–induced craving and anxiety were evident soon after acute exposure to the drug (session 1). In addition, there was a protracted effect on these measures 1 week after short-term repeated administration of CBD (session 4). CBD also tended to reduce physiological measures of stress reactivity, such as increased heart rate and cortisol levels that were induced by salient drug cues. Importantly, CBD administration (at 400 mg or 800 mg) was associated with only mild adverse events.
This clinical trial was designed to assess the acute, short-term, and protracted consequences of CBD administration. The strongest effects were apparent during the first (acute) session. However, this was the session in which the cues and laboratory setting were novel. Indeed, there was a decrease in cue-induced craving across sessions in individuals receiving placebo, whereas the low craving levels observed in CBD-treated participants remained stable throughout the study. This observation is perhaps not unexpected, as habituation of cue- and stress-induced responses (including craving and physiological measures) can occur over time in laboratory settings. Such habituation notwithstanding, the capacity of CBD to reduce craving and anxiety 1 week after the final administration (and without daily exposure to the laboratory setting) mirrors the results of the original preclinical animal study (
15), suggesting that the effects of CBD are long lasting, even when the cannabinoid would not be expected to be present in the body. We did not conduct toxicological measures of CBD concentrations in this study, but our previous pharmacokinetic analysis (
8) did not detect CBD in plasma 1 week after a single administration of 400 mg or 800 mg; however, unpublished data indicated that low levels of CBD metabolites in urine were evident in some participants in that study. Irrespective of potential trace metabolite levels, the results of the present study highlight the fact that the effects of CBD on neural systems relevant to craving and anxiety persist in the absence of high pharmacological concentrations. A recent animal study also confirmed the prolonged effects of CBD on drug (alcohol and cocaine) seeking and anxiety-like behaviors 5 months after its short-term administration (
29). This protracted property of CBD would have significant clinical implications, especially for patient populations in which daily medication adherence may be challenging.
The effect of CBD on cue-induced craving was not reflected in the at-home, general craving self-reported Heroin Craving Questionnaire scores, in which no significant effects were evident. Several explanations could account for this difference, especially considering that the craving methods reflect different environmental factors and types of craving; that is, general craving experienced at home compared with craving triggered by drug-associated stimuli in the study sessions. CBD has been shown in clinical (
23,
30) and preclinical (
15,
29) studies to be most sensitive to cue stimuli, which, together with the results from the present study, suggests that CBD may be relevant to the attentional saliency of drug cues that are associated with craving contributing to relapse (
31).
The potential anxiolytic properties of CBD have been documented in previous clinical studies (
20,
21). Moreover, in vivo neuroimaging has indicated that CBD blunts activity in limbic neural circuits engaged during negative emotional processing (
32) and modulates networks linked with attentional salience processing (
33). The results of numerous animal models have also reinforced the anxiolytic role of CBD (
34,
35). The observation that CBD decreased cue-induced cortisol levels in the present study is consistent with its reducing negative stress or anxiety states, which is highly related to amygdala reactivity (
36). No clinical study has yet reported an effect of CBD on a depressed emotional state, and CBD did not reduce PANAS negative affective scores increased by drug cues in the present study. Although a significant body of research remains to be conducted on CBD, its potential significance for ameliorating features central to substance use disorders may be related more to craving and anxiety.
Regarding our secondary outcome measures, cognition was not improved under the acute or short-term conditions of CBD administration examined, consistent with a recent investigation in which a single CBD administration also failed to improve verbal or spatial working memory during tobacco abstinence (
37). Disparate results exist regarding the effects of CBD on cognition, likely because of the varied neuropsychiatric disorders (e.g., schizophrenia or substance use disorder) and treatment conditions under which it has been explored. Additional studies with long duration of treatment are necessary to evaluate the potential effects of CBD on cognition.
Several aspects of this study should be considered for the interpretation and generalizability of the results. Our two primary outcomes, craving and anxiety, were subjective, self-reported measures, which may have introduced unreliability or bias. Nevertheless, the subjective effects reported by participants receiving placebo—particularly in session 1, in which the strongest craving was induced by the drug cue (but not the neutral cue, emphasizing the specificity of the subjective measures)—paralleled the objectively measured physiological responsivity of heart rate and cortisol levels. In session 1, CBD blunted the cue-induced increases in these physiological measures, substantiating the participant self-reports. The small sample size did not allow for a thorough evaluation of sex effects, an important evaluation given that women typically have higher craving and anxiety than men. Moreover, interparticipant variability was likely in this study owing to the high lipophilicity and complex metabolism of CBD. Because no CBD toxicology tests were conducted, we cannot address interparticipant metabolic variability, but a dose-dependent pattern was observed for many of the variables studied, with the highest dose (800 mg) often leading to the strongest outcomes. Another limitation of the study was the potential use of cannabis outside the laboratory setting. Indeed, one participant in the placebo group had positive THC or cannabinoid toxicology test results and admitted to cannabis use. Urine THC or cannabinoid was also detected among the participants in the CBD groups, but only in sessions immediately after CBD administration, supporting those participants’ denials of recent cannabis use. Other factors to consider for the toxicology findings include the potential cross-reactivity of the assay, the fact that the CBD used in the study (Epidiolex) has trace amounts of THC (
17), and the possibility that oral CBD converts to THC with highly acidic gastric conditions in vitro (
38), although this conversion is not supported by published in vivo evidence (
17,
39). During the course of the study, no one in the 800 mg group used opioids, one participant in the 400 mg group used heroin, and two participants in the placebo group relapsed to heroin use. Long-duration treatment studies are needed to fully address the effects of CBD on relapse prevention.
In summary, the potential of CBD to reduce cue-induced craving and anxiety, along with its safe pharmacological profile, low mortality risk, and lack of hedonic properties, indicates that this phytocannabinoid holds significant promise for treating individuals with heroin use disorder. A successful nonopioid medication would add significantly to the existing addiction medication toolbox to help reduce the growing death toll, enormous health care costs, and treatment limitations imposed by stringent government regulations amid this persistent opioid epidemic.