Reward system dysfunction (deficits in reward learning and reduced response to gains in the reward network of the brain) appears to be concurrently and prospectively related to depression in adults and in adolescents. Blunted responses in the striatum (caudate, putamen, nucleus accumbens) to rewarding stimuli are found in depressed individuals (
1,
2), predict later depressive episode (
3–
5), and are found in the offspring and first-degree relatives of depressed individuals (
6,
7). This suggests that reward system dysfunction may serve as a candidate neural correlate or even risk factor for depression. Our goal in this study was to examine the relationships between regions of a cortico-striatal circuit supporting reward processing and both current depression and cumulative depression history in a sample of adolescents who have been participating since preschool in a longitudinal study of early-onset depression.
In addition to being present in currently depressed individuals, blunted reward responses have been reported in studies of patients in remission from depression, both behaviorally (
8) and in the ventral striatum (
9). However, brain responses to reward occur in regions beyond the striatum, engaging a broader cortico-striatal circuit. Individuals in remission show blunted neural responses to reward in this broader circuit, including in the anterior cingulate cortex (ACC) (
10–
12) and the insula (
13). Such findings suggest the possibility that hyporeactivity of a broader set of regions within the cortico-striatal circuit persists beyond the depressive episode, potentially putting patients at risk of recurrence, particularly with early-onset depression. Specifically, subcortical striatal function may be disrupted concurrently with depression. If so, such striatal disruption in early-onset depression may in turn contribute to disruption in the development of cortical regions and their functions. In later-onset depression, these cortical areas are more developed, and thus their function may be less disrupted by depression. Few studies have been able to simultaneously examine both current depression severity and past history of depression (particularly using prospective data) to test this hypothesis. Thus, our longitudinal sample presents a unique opportunity for testing the prediction that current depression is associated with a more focal blunted response to rewards in the striatum, while previous or cumulative depression (especially early-onset depression) is associated with a more global blunted response across the cortico-striatal circuit.
Furthermore, there is evidence that depression can be related to dysfunction in both reward anticipation and receipt. Blunted reactivity to rewarding cues (anticipation) and outcomes (receipt) has been associated with depression in adolescents (
1,
2,
6,
14–
16), suggesting deficits in the experience of rewards or hedonic tone. However, other studies have observed blunted responsivity only to reward anticipation (
5,
7,
17,
18). Therefore, determining whether depression is more strongly related to reward anticipation or receipt will help clarify the neural and behavioral mechanisms that contribute to reward processing dysfunction related to depression.
We tested these predictions using functional MRI (fMRI) responses in regions of the brain’s reward system (e.g., cortico-striatal network) to monetary rewards and losses in a longitudinal sample of adolescents who have been annually assessed for psychiatric symptoms since early childhood. We examined neural responses related to both current depression severity and cumulative depression severity since early childhood. Because participants were followed longitudinally, we had measures of depression severity prospectively acquired at annual assessment waves using clinical interviews rather than based on retrospective report. Furthermore, while previous studies have typically compared healthy control subjects with depressed patients as a group (
1,
2,
14,
16), in this study we used continuous measures of depression severity, which are more reliable than categorical measures of psychopathology (
19) and are in line with the Research Domain Criteria framework (
20,
21). We also tested the hypothesis that early onset of depression would be associated with blunting in a broader network of reward-responsive regions as compared with only current depression by comparing the relationship between cortico-striatal activation and depression symptoms reported at assessment waves during three different developmental periods: preschool, school age, and adolescence.
Results
Group-Level Response to Reward Anticipation and Receipt
Participants showed significant BOLD response to reward anticipation and receipt across regions of the cortico-striatal circuit, including the dorsal and ventral striatum, dorsal and rostral anterior cingulate cortex (dACC and rACC, respectively), and insula, among other regions (p<0.01 corrected; see Figure S2 in the online supplement).
Depression Severity and Neural Response to Reward Receipt
Neither cumulative nor current depression was related to mean activation to reward receipt across the cortico-striatal circuit or in specific individual regions of interest (see Table S1 in the online supplement). Furthermore, whole brain analyses did not reveal any significant clusters of activation during reward receipt that were correlated with cumulative depression severity. Some clusters were correlated with current depression severity, although none were in the dorsal or ventral striatum (see Table S2 in the online supplement).
Depression Severity and Neural Response to Reward Anticipation
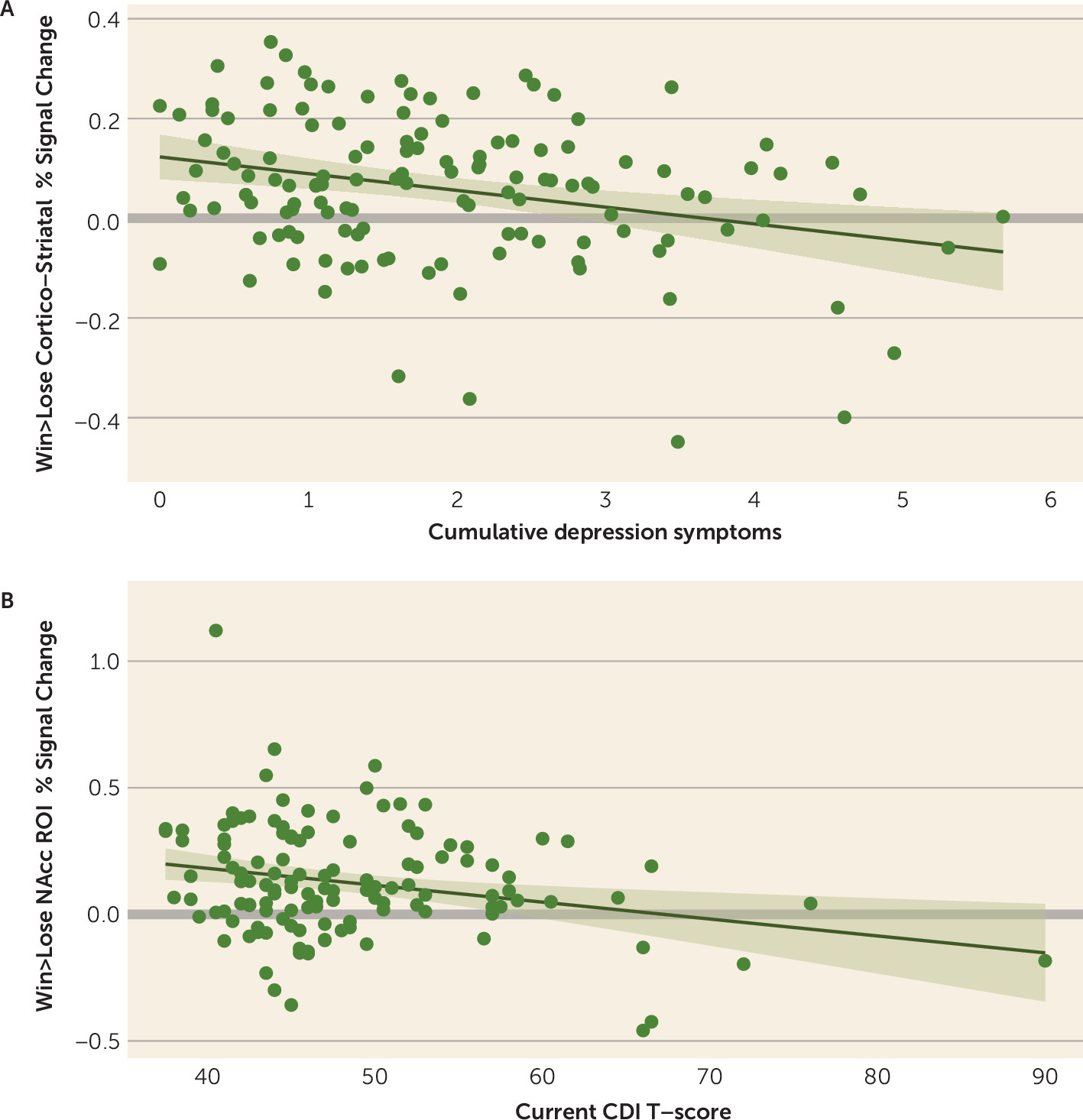
Multiple regression analyses that included both current and cumulative depression as simultaneous regressors showed that cumulative depression was related to mean activation in the cortico-striatal circuit (
Table 2;
Figure 1A), as well as the caudate, putamen, insula, dACC, and rACC. Current depression was related to activation in the nucleus accumbens (
Figure 1B) and dACC (
Table 2). The relationship with the nucleus accumbens was nominally significant but not after false discovery rate correction, although this relationship was significant when cumulative depression was excluded from the model and false-discovery-rate corrected (see Table S3 in the
online supplement). The association with the dACC did not hold when cumulative depression severity was excluded from the model (see Table S3). Regressions with current and cumulative depression as separate regressors showed a similar pattern of results (see Table S3). Findings were consistent when the analyses accounted for psychotropic medication use and for the subsample followed since preschool (see Tables S5–S7, S9, and S10 in the
online supplement). See the
online supplement for complementary analyses using multivariate analyses of covariance with all four cue-type conditions.
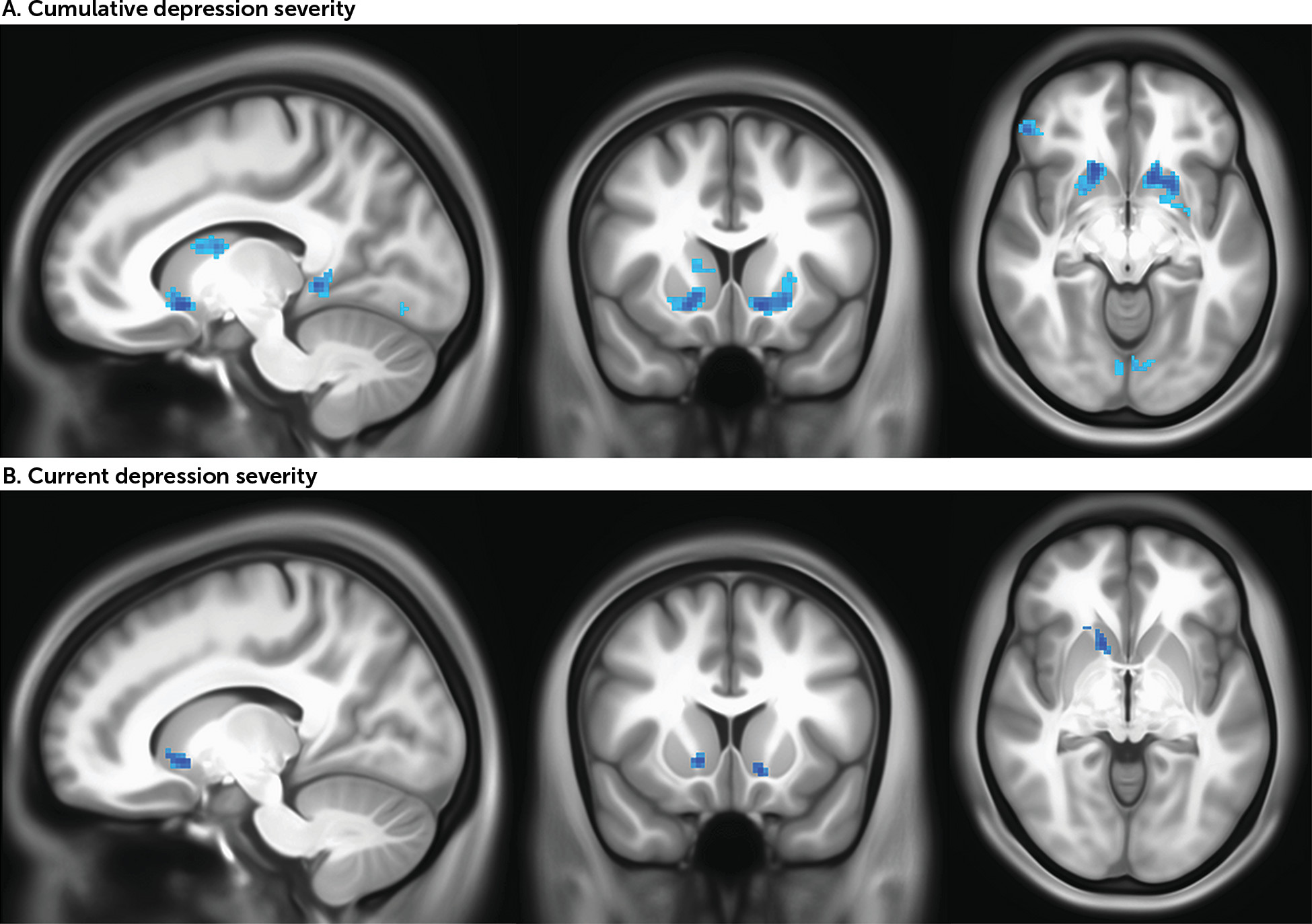
In whole brain analyses, consistent with the region-of-interest analyses, cumulative depression was negatively correlated with activity in the dorsal and ventral striatum as well as cortical regions including the left insula and left and right superior frontal gyrus (p<0.005, uncorrected for spatial extent) (
Figure 2A,
Table 3). Results from ETAC analyses confirm the significance of striatal regions of activity (see Figure S7 and Table S12 in the
online supplement). The results did not meaningfully differ when the analyses accounted for psychotropic medication (see Figure S5B and Table S8 in the
online supplement). For the subsample followed since preschool, a mask was applied using the clusters from the full sample, and analyses tested whether activation within this mask correlated with cumulative depression. All eight clusters were negatively correlated with cumulative depression, including clusters in the dorsal and ventral striatum (see Figure S6B and Table S11 in the
online supplement).
In whole brain analyses, consistent with the region-of-interest analyses, current depression was negatively correlated with activity in the ventral striatum in the full sample (p<0.005, uncorrected for spatial extent) (
Figure 2B,
Table 3). Four cortical clusters located in the left middle occipital gyrus, left middle frontal gyrus, right inferior temporal gyrus, and left medial frontal gyrus were also positively correlated with current depression. Results from ETAC analyses confirm the significance of striatal regions of activity (see Figure S7 and Table S12 in the
online supplement). The results did not meaningfully differ when the analyses accounted for psychotropic medication (see Figure S5A and Table S8 in the
online supplement). In the subsample followed since preschool, three clusters were negatively correlated with current depression, two in the striatum (the left and right ventral striatum) and one in the left ventral pons (see Figure S6A and Table S11 in the
online supplement).
Depression Severity During Distinct Developmental Periods and Response to Reward Anticipation
As shown in Table S3 in the online supplement, after false discovery rate correction, regression analyses with each developmental period as a separate regressor indicated that preschool depression severity was related to reduced activity in the cortico-striatal circuit as well as in the caudate, putamen, insula, dACC, and rACC. School-age depression severity was related to reduced activity in the cortico-striatal circuit, caudate, and putamen. Adolescent depression severity was related to reduced activity in the cortico-striatal circuit and the nucleus accumbens. The results were mostly consistent when the analyses accounted for psychotropic medication use and for the subsample followed since preschool (see Tables S7 and S10 in the online supplement).
We then directly compared each developmental period by conducting multiple regression analyses that simultaneously included preschool, school-age, and adolescent depression severity as regressors. Because these models exclude participants for whom depression severity ratings were missing from any of the three developmental periods, only the subsample that was followed since preschool was used. As shown in Table S4 in the online supplement, preschool depression severity was related to reduced activation in the cortico-striatal circuit, putamen, and the rACC. In contrast, adolescent depression severity was related to reduced activation only in the nucleus accumbens. The results were consistent when the analyses accounted for psychotropic medication use (see Table S6 in the online supplement).
Discussion
We replicated previous findings (
5,
18,
44) that greater current depression severity was related to reduced activity in the nucleus accumbens (i.e., ventral striatum) to reward anticipation, but not receipt, in both a priori region-of-interest and whole-brain analyses. A priori region-of-interest analyses further revealed that cumulative depression severity was related to blunting to reward anticipation across the cortico-striatal circuit, with whole brain analyses also showing that cumulative depression severity was related to blunted activity in both the dorsal and ventral striatum. Of note, the whole brain associations did not survive very conservative whole brain correction, primarily because of the large spatial extent threshold mandated by such corrections. Supplementary analyses using equitable thresholding and clustering were used to assess for the presence of smaller, more intense clusters. The strongest results were directly consistent with the region-of-interest-based analyses in showing a relationship between current depression severity and ventral striatum activity, with broader relationships of cumulative depression across the ventral and dorsal striatum. Additional analyses demonstrated that preschool depression severity was related to blunted response to anticipation in regions including the dorsal striatum (i.e., putamen) and rostral ACC, while adolescent depression severity was related to response in the ventral striatum (i.e., nucleus accumbens). Finally, we did not find support for any a priori relationships between depression severity and response to reward receipt.
Our finding that current depression severity was associated with focal dysfunction in the ventral striatum while cumulative depression severity was associated with global dysfunction of the cortico-striatal circuit has implications for future research seeking to identify risk factors and consequences of depression. First, global blunting of the cortico-striatal circuit could represent a risk factor for chronic or recurrent depression and may be associated with early onset and/or more severe forms of depression. This hypothesis is consistent with our finding that both cumulative depression severity and depression severity in the preschool period specifically were associated with reduced responsivity across the cortico-striatal network. This hypothesis would predict that children who go on to experience early onset of depression or more severe or chronic depression will show more widespread dysfunction of a cortico-striatal network related to reward anticipation even before the onset of depression.
A second possibility is that blunting across a broad cortico-striatal network represents a scar of chronic depression. That is, with repeated exposure to depressive symptoms, particularly early in life, the cortico-striatal circuit may not develop properly, giving rise to deficits in reward processing. Further, our findings also demonstrated that current depression severity was associated with hyporeactivity of the ventral striatum to anticipation of reward. If such an association between current depressed mood state and ventral striatal hyporeactivity to reward anticipation is present across development, repeated experience of depression that starts early in childhood could lead to downstream hyporeactivity of the entire circuit. An early onset of depression may disrupt this network as the child is developing and have a cascading and broader impact. This could be tested by longitudinal studies concurrently measuring depression and neural responses to rewards from early childhood to adolescence.
One intriguing possible explanation for these findings is that, over time, blunting to rewards transitions from the ventral to the dorsal striatum, becoming a “habit” of reduced reward response. This is analogous to the hypotheses of Everitt and Robbins et al. proposing stages of substance use. They proposed a shift in responsivity from the nucleus accumbens to the dorsolateral striatum as drug-seeking behavior transitions from instrumental (i.e., controlled or voluntary) to habitual, via striato-nigro-striatal ascending anatomical connections (
45,
46), with ventral areas of the striatum innervating more dorsal areas via spiraling anatomical connections (
47,
48). The authors further posit that the striatum may interact with cortical regions to drive negatively reinforced behaviors, resulting in anhedonia (
45). The present findings lend some evidence to this theory’s generalizability to depression, with a greater accumulation of depression symptoms associated with more blunted activity in the dorsal striatum and cortical regions and acute depression more associated with blunting of the ventral striatum. If the findings are replicated, this hypothesis has implications for how we characterize and treat depression.
Finally, our findings revealed associations between depression and reward anticipation but not reward receipt, contrary to our hypotheses. While some studies have found blunted reactivity to both reward anticipation and receipt (
1,
2), others have similarly found stronger relationships between depression and reward anticipation, sometimes to the exclusion of reward receipt (
7,
17,
18), including a study of 1,576 adolescents (
5). Moreover, anhedonia appears to be linked more strongly to blunted responses to reward anticipation than to reward receipt (
49). This dissociation has interesting implications. While blunted reactivity to reward receipts may be representative of hedonic pleasure, blunted reactivity to reward anticipation may represent deficits in learning about cues that predict reward or in ability to represent future reward experience or information. Our findings suggest the possibility that rather than leading to failure to experience pleasure from rewards, chronic depression experienced over childhood affects motivation more than hedonic response in adolescence. One possible explanation is that reward anticipation is a developmental skill honed by learning and experience, whereas hedonic response to rewards is more automatic. If so, repeated exposure to depression during development may disrupt this trajectory, resulting not in impairments to hedonic response to rewards per se but rather in altered motivation and related aberrant reactivity of the striatum and broader regions. In fact, other studies have found that depression is related to reduced behavioral reward learning (
50) and reward-related decision making (
51). Further studies assessing reward learning and responsivity to reward cues longitudinally will be necessary to further test this hypothesis.
A positive relationship between current depression and medial/middle prefrontal regions appeared in exploratory whole brain analyses, as has been observed in previous studies (
2). One possible explanation is that depressed individuals use cognitive resources to compensate for insufficient striatal resources in order to represent the reward that follows the cue (i.e., learn the cue). This would suggest coordination between the cortico-striatal circuit and frontal regions in learning and anticipating rewards.
Several limitations of this study should be noted. First, a subset of participants included in the full sample (N=20) were added at later waves to increase the sample size of healthy control subjects for the imaging waves and therefore were missing depressive measures before age 9. The findings did not meaningfully change when this subset was excluded. Second, because rewards in the fMRI task we used were based on chance, we were not able to compare neural response to reward with a behavioral measure of reward learning. At the same time, the task we used avoids potential confounders that can occur with such behavioral measures, such as motor preparation between the anticipatory cue and action or task anxiety over one’s performance (
52). Third, our whole brain results were significant at a p value of 0.005 but did not meet the full spatial extent mandated by a conservative whole brain correction. However, for the striatum, such a large spatial extent may not be realistic. Whole brain results using equitable thresholding and clustering confirm the striatal results found in a priori region-of-interest-based results. Fourth, our a priori planned analyses focused on win>lose contrasts, and complementary multivariate analyses of covariance with additional cue type conditions were very consistent, but not every result replicated to the same significance level (see the
online supplement). Fifth, we used mean BOLD signal to measure cortico-striatal circuit activity. An interesting extension of these findings will be to test whether the functional connectivity of these regions is related to depression severity. Finally, it is often difficult to disentangle chronicity from developmental effects. Children with early-onset depression are at greater risk of experiencing more chronic depression throughout childhood, making it difficult to know whether associations between cumulative depression and cortico-striatal function is the result of early-onset depression or of a chronic course. However, our finding that nucleus accumbens activity was most associated with adolescent depression severity provides some evidence for developmental specificity. Future studies comparing youths with depression exclusively in early childhood with those who experience depression exclusively later in life could inform such questions.