Most older adults experience deterioration in cognitive function (
1,
2). This age-related cognitive decline varies among individuals, with individual differences related to preclinical Alzheimer’s pathology, cerebrovascular disease, education, and lifestyle (
3). Age-related cognitive decline can have a negative impact on quality of life, interpersonal relationships, and capacity for making decisions about finances, health care, retirement, and other issues important to older adults (
4).
One tool for addressing cognitive decline is cognitive training (
5). This therapeutic procedure typically relies on activation of neural circuitry known to be impaired in illness, at risk of decline, or compensatory for other cognitive functions. Neuroplasticity is achieved through repetitive drill and practice exercises that require participants to perform cognitive operations that are slightly above their current ability threshold. Several studies find support for its use in age-related cognitive decline (
6). For example, the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, a large randomized trial of a cognitive intervention in an older population with normal cognitive ability, showed that cognitive training has beneficial effects, lasting at least 5 years, on specific functions (
7). Cognitive training also shows benefits in cognitively compromised populations, including those with mild cognitive impairment, dementia, major depression, and schizophrenia (
8,
9). However, concerns with cognitive training research include a small overall effect size and limited evidence of transfer effects to everyday cognitive tasks (i.e., improvement in “functional cognition”) (
10).
Several pharmacological therapies have been tried for memory enhancement, but no treatment is currently approved for age-related cognitive decline. Trials have included medications typically used for Alzheimer’s disease (
11), antidepressants (
12), and nutritional supplements (
13). Vortioxetine is a medication that is approved for the treatment of major depression. Unlike most other serotonin reuptake inhibitors, vortioxetine is a potent antagonist of postsynaptic 5-HT
3 and 5-HT
7 receptors, an effect that has been proposed to indirectly increase dopaminergic, cholinergic, and histaminergic transmission, which are involved in cognitive function (
14). Preclinical research demonstrated vortioxetine’s procognitive effects, putatively related to these postsynaptic receptor effects (
14). It has shown both subjective and objective cognitive benefits in clinical trials in depression. It was shown to be superior to placebo and to an active control for improved neuropsychological functioning in older adults with major depression (
15) and in working adults with major depression (
16), and a recent neuroimaging study found that vortioxetine has effects on the neural circuitry supporting cognitive function (
17). These procognitive effects were independent of vortioxetine’s effect on depressive symptoms.
The rationale for combining vortioxetine with a cognitive training program is to improve the cognitive abilities of older adults to a greater degree than with training alone (
18). Vortioxetine in combination with cognitive training could robustly drive beneficial plasticity of the aging brain, resulting in significant improvement in memory and executive function in older adults, thereby remediating age-related cognitive decline. Both of these interventions have shown limited success in transfer to using cognition in everyday tasks (i.e., “functional cognition”) in healthy adults.
Therefore, we tested the efficacy of vortioxetine added to a cognitive training program to remediate age-related cognitive decline in a randomized clinical trial. We randomly assigned 100 participants age 65 or older with age-related cognitive decline to receive either vortioxetine or placebo while also undergoing computerized cognitive training for 6 months. We hypothesized that participants assigned to receive vortioxetine plus cognitive training would show a greater improvement in global cognitive performance on a battery of memory and executive function measures, as well as in functional cognition, compared with those assigned to receive placebo plus cognitive training.
Methods
This was a randomized controlled clinical trial with two parallel groups (vortioxetine and placebo; both groups received computerized cognitive training) and blinded outcome assessments. The study was approved by Washington University’s institutional review board. Potential participants were enrolled after providing university-approved written informed consent.
Participants
From August 2016 to July 2018, community-dwelling adults age 65 or older were recruited through Washington University’s research participant registry and public advertisements in the St. Louis region.
Inclusion criteria were age 65 or older and age-related cognitive decline as defined by 1) self-reported cognitive dysfunction that is attributed to the aging process (in response to screening questions) and 2) scoring within one standard deviation of the age-matched mean score on the NIH Toolbox Cognition Battery Fluid Cognition Composite both at baseline and after the 2-week cognitive training lead-in. Both a lower limit (to exclude dementia and mild cognitive impairment) and an upper limit (to avoid ceiling effects) were used. An upper limit of “at, but not above“ age-matched norms reflects the fact that with typical aging, older adults have declines in the domains of memory, executive functioning, and information processing speed compared with younger cohorts (and thus have age-related cognitive decline).
Exclusion criteria were presence of known dementia or other clinical neurodegenerative illness (e.g., Parkinson’s disease, cerebrovascular disease) per self-report or medical records; current major depressive episode, current or past mania or hypomania, lifetime psychotic symptoms, current alcohol or substance use disorder, and generalized anxiety disorder (these psychiatric exclusions were used to clarify whether vortioxetine’s procognitive effects are secondary to antidepressant or related effects); medical conditions that suggest shortened lifespan, such as metastatic cancer, or that would prohibit safe participation, such as narrow-angle glaucoma (a relative contraindication to vortioxetine); sensory impairment that would prevent participation; estimated IQ <70, as measured by the Wechsler Test of Adult Reading (
19); alcohol or substance abuse within the past 6 months; concurrent cognitive training, such as brain-training software, or other interventions expected to affect neuroplasticity; psychotropic medications or those with likely CNS effects (with the exception of low-dose trazodone at night for sleep) or any drug that interacts pharmacokinetically with vortioxetine.
Cognitive training lead-in phase.
All participants received cognitive training using a well-validated software program, “Scientific Brain Training Pro” (
20–
22). Prior to randomization, all participants received 2 weeks of cognitive training (via their home computer), five times weekly for 30 minutes a day. This cognitive training lead-in was a key design feature similar to augmentation designs in depression and other CNS clinical trials (
23). The lead-in phase allowed for an examination of participants’ motivation, willingness, and ability to undergo cognitive training. Those unable or unwilling to do so, as well as those who reached more than one standard deviation above normal on the NIH Toolbox Cognition Battery Fluid Cognition Composite after 2 weeks of cognitive training, were removed from the study prior to randomization.
Cognitive training program.
Cognitive training was delivered in a protocolized format in which 25 different cognitive exercises are made available for the participants. Training progresses from basic (i.e., processing speed, attention) to more complex (i.e., working memory, executive functions) cognitive functions. Each cognitive training exercise has 30 levels of difficulty, which change adaptively by increasing after consecutive trials of 80% success or better and decreasing after consecutive trials of 70% success or lower. Automated feedback is provided after each trial, and participants have access to visual displays of their progress on each task throughout their training period.
For the standardized psychoeducation and motivation of participants, training of study staff was conducted, and fidelity assessed, by an expert in cognitive training (C.R.B.), following a standardized manual. Trainers (bachelor’s-level individuals) met with each participant for approximately 1 hour to demonstrate the program, explain the task purpose and instructions, and provide the training schedule (a target of 150 minutes per week). This included psychoeducation on the purpose of improving cognition, which is integrated into the participant’s own profile of cognitive strengths and limitations and self-defined goals for functioning. A manual provided participants with clear instructions, goals, and strategies for each of the exercises. The amount of effort participants put into the program was emphasized rather than level achievement.
Trainers provided motivational calls to participants who had not logged on to the program in at least 3 consecutive days and those who logged less than 120 minutes in the program (80% of the target number) for the week (unless they were known to be on vacation or had a family emergency or some other factor that prevented their engagement). Motivational calls were also utilized to encourage variety if a participant appeared to be using only the exercises for one cognitive domain. Motivational calls primarily consisted of a reminder of the target time (150 minutes per week) and why meeting the target is important (to challenge cognition and maintain any gains achieved), as well as a reminder to contact study staff if the participant was experiencing any technical difficulties or had any questions. There was no significant difference in the number of motivational calls provided between treatment groups (placebo group: mean=1.57, SD=2.21; vortioxetine group: mean=1.76, SD=2.46; t=0.41, df=98, p=0.68).
Randomization.
The randomized phase was 26 weeks long, during which participants self-administered a tablet containing either 10 mg of vortioxetine or placebo. All participants received cognitive training.
The study statistician generated the random allocation sequence, preset to allocate 50% to each group. Enrollment of participants and allocation to study conditions were conducted by study staff. Randomization was blocked within strata using random permuted blocks, and randomization assignment was concealed by using drug and placebo tablets of matching appearance.
The study team monitored and supported participants’ adherence to both the cognitive training program and the study medication. The cognitive training program has built-in adherence monitoring, and study staff also provided motivational calls. As noted above, motivational feedback included assistance with any technical difficulties, explanations of instructions, and reminders of weekly total minutes, in order to get training time at least up to 80% of the target of 150 minutes per week.
Vortioxetine was initiated at 10 mg, with no titration period. We examined medication adherence via self-report and pill count. The clinician who prescribed the medication (E.J.L.) is a board-certified geriatric psychiatrist whose only contact with participants was at study baseline, during the assessment for inclusion in the study.
Schedule of assessments.
Our primary outcome measure was the NIH Toolbox Cognition Battery Fluid Cognition Composite. The NIH Toolbox Cognition Battery is a computer-based instrument assessing five cognitive subdomains, measuring both crystalized and fluid cognition. Unlike tests such as the Mini-Mental State Examination and the Montreal Cognitive Assessment, which screen for global cognitive impairment, the Fluid Cognition Composite is specific to fluid abilities. These abilities are used throughout life to solve problems, think and act quickly, and adapt to new situations in everyday life, and they correlate strongly and negatively with age (
24) and are therefore highly relevant to age-related cognitive decline. The Fluid Cognition Composite is based on five measures of global cognitive performance: the Flanker Inhibitory Control Test (measuring attention and inhibitory control), which requires that participants focus on a given stimulus (an arrow) while inhibiting attention to the stimuli flanking it; the Dimensional Change Card Sort Test (measuring cognitive flexibility), which asks participants to match a series of bivalent test pictures to the target pictures, switching between the dimensions of color and shape; the List Sorting Working Memory Test, which has participants order objects (either food or animals) in size from smallest to largest, after which they are then presented with both food and animals and asked to report food in size order then animals in size order; the Picture Sequence Memory Test, an episodic memory test in which participants recall a series of illustrated objects and activities that increases in length and is presented in a particular order on the screen; and the Pattern Comparison Processing Test (measuring processing speed), which asks participants to discern whether two side-by-side objects are the same or not, in a 90-second period (
24).
This cognitive battery was carried out at the beginning of the lead-in phase (to establish a pretraining baseline and to clarify inclusion in the study), at the beginning of the randomized phase, and at 4, 12, and 26 weeks after randomization. We used the age-corrected standard score. As a secondary outcome measure, we assessed functional cognition using the UCSD Performance-Based Skills Assessment (UPSA), at randomization and at 26 weeks. The UPSA is a validated test that uses role play and props that require participants to demonstrate their competence to perform everyday functioning tasks in domains such as comprehension and planning, finance, transportation, and communication. All outcomes were measured by assessors blind to treatment condition.
Safety and adverse events.
We assessed all participants before randomization with medical history, physical examination, and routine safety laboratory tests (electrolyte levels, liver and kidney function, thyrotropin levels). We assessed vital signs at all in-person visits and, after randomization, assessed for study medication side effects at all visits by asking participants if they had experienced any problems since their last visit. We assessed for and recorded adverse events throughout the trial.
Other assessments.
At baseline, we confirmed absence of psychiatric illness with the Structured Clinical Interview for DSM-5 (
25) and demonstrated absence of current depressive and anxiety symptoms with the Patient Health Questionnaire–9 (PHQ-9) (
26) and the Penn State Worry Questionnaire–Abbreviated (
27).
Statistical Analysis
Study data were managed using REDCap (
28). Analyses were performed using R, version 3.5.2 (
https://cran.r-project.org), and SAS, version 9.4. (SAS Institute, Cary, N.C.). All reported p values are two-tailed, with the significance threshold for all tests set at ≤0.05. The primary efficacy analysis addressed neurocognitive changes and the secondary efficacy analysis addressed functional changes.
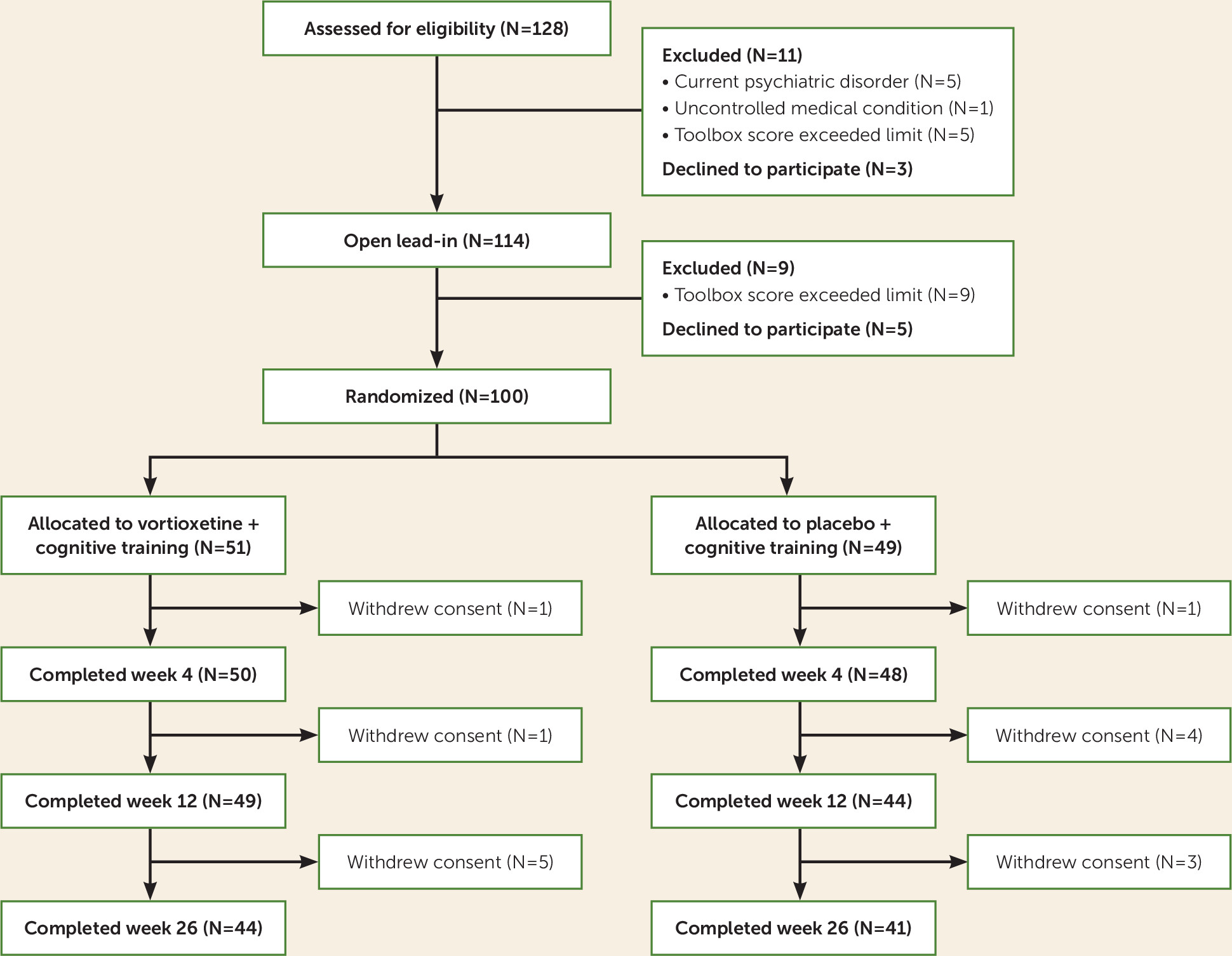
For a simple t test, the sample size of 100 with 80% power (at two-tailed p<0.05) allowed the detection of a moderate effect size (d=0.55) for greater cognitive improvement in the vortioxetine group compared with the placebo group. We used the intent-to-treat principle in examining vortioxetine’s efficacy: all randomized participants were included. Because 15 participants withdrew consent after randomization (
Figure 1), the PROC MIXED procedure in SAS used 376 observations (94%) for the primary analysis and 185 observations (92.5%) for the secondary analysis. Our analytic strategy was a restricted maximum likelihood–based mixed model for repeated measures (MMRM) approach, where treatment-by-week interaction (from baseline to posttreatment assessment) was the key analysis in a model including week, treatment group, and treatment-by-week interaction. When the treatment-by-week interaction was significant, contrasts were used to test comparisons of the mean change over time between the two treatment groups. The differences in mean change over time between treatment groups were estimated on the basis of the least square means for the treatment-by-week interaction in the MMRM model. With this approach, the primary analysis was the mean change from baseline to week 4, week 12, and week 26 in NIH Toolbox Cognition Battery Fluid Cognition Composite score; the secondary outcome measure was the mean change in UPSA score from baseline to week 26.
To examine adherence to online cognition training, we used the t test to compare the mean difference in total cognition training time from week 0 to week 26 between the vortioxetine and placebo groups. For the safety evaluation, the analysis was based on all randomized participants who had at least one self-reported adverse event. We used Fisher’s exact test to compare the proportions of adverse events between the vortioxetine and placebo groups.
Results
As shown in
Figure 1, we screened 128 age-eligible individuals for study eligibility; of these, 11 were ineligible, three declined to participate, and 114 entered the study. Of these, five withdrew consent before completing the 2-week open lead-in phase, and nine were excluded because they had scores above the predetermined ceiling of one standard deviation above age-matched norms, leaving 100 participants randomized—51 to receive vortioxetine plus cognitive training, and 49 to receive placebo plus cognitive training.
Table 1 summarizes the participants’ baseline characteristics.
Primary Outcome Measure: Global Cognitive Performance
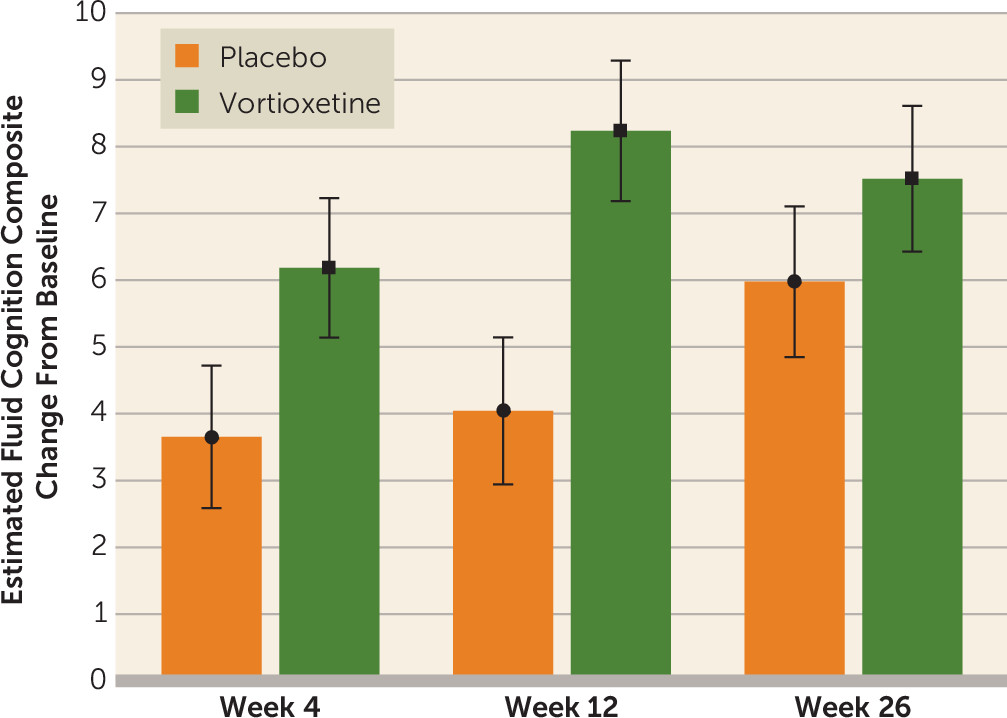
Figure 2 shows changes in scores on the NIH Toolbox Cognition Battery Fluid Cognition Composite over the 26 weeks of randomized treatment (see also Figure S1 in the
online supplement). The vortioxetine plus cognitive training group had greater improvement in cognitive function than the placebo plus cognitive training group. Of the three individual assessment points (weeks 4, 12, and 26), a significant separation of vortioxetine from placebo was observed only at week 12. Between-group differences in the individual Toolbox Cognitive Battery tests were statistically significant only for the Dimensional Change Card Sort at week 12 (see Table S1 in the
online supplement).
Secondary Outcome Measure: Functional Cognition
Both groups showed significant improvement on the UPSA from baseline to week 26 (vortioxetine group: increase in score, mean=3.22, SD=1.19; placebo group: increase in score, mean=1.39, SD=1.23). The changes did not differ significantly between the two groups (p=0.29).
Safety and Tolerability
Table 2 summarizes adverse event data in the vortioxetine and placebo groups. Nausea was significantly more common in the vortioxetine group.
Treatment Guess
For participants assigned to receive vortioxetine, the treatment guess was 63% vortioxetine (N=32) and 37% placebo (N=19). The proportions were not significantly different for participants in the placebo group, among whom the treatment guess was 67% vortioxetine (N=33) and 33% placebo (N=16).
Online Cognitive Training Adherence
There was no significant difference in the mean of total training time between the vortioxetine and placebo groups (3,673.5 minutes [SD=1,506.3] compared with 3,457.3 minutes [SD=1,376.5], p=0.456). (Figure S2 in the online supplement depicts cognitive training adherence over time in both groups.)
Discussion
This randomized trial evaluated the effects of combining vortioxetine, a putative procognitive and proneuroplastic medication, with computerized cognitive training for older adults with age-related cognitive decline. Our main finding is that the combination of vortioxetine with cognitive training showed greater improvement in global cognitive performance compared with cognitive training with placebo. The groups separated in efficacy at the week-12 assessment time point only. This treatment combination was feasible and well tolerated, and participants carried out this combined intervention with generally good adherence to both the medication and the training. We attribute the positive effects to synergistic procognitive actions of vortioxetine and cognitive training. Alternatively, the two interventions simply had additive procognitive effects. These findings are important because this is the first study, to our knowledge, to demonstrate that a putative procognitive drug could be combined with cognitive training in age-related cognitive decline to provide a greater improvement than can be achieved by cognitive training alone.
This clinical trial was specifically designed to test the procognitive benefits of a pharmacological agent when combined with cognitive training. Therefore, it included a lead-in cognitive training phase to clarify whether participants could engage in cognitive training; the subsequent randomized phase demonstrated good adherence and relatively low dropout. This lead-in phase also repeats the cognitive outcome assessment prior to randomization and therefore reduces the potential confounder of practice effects, which occur in treatment studies that use cognitive or other performance assessments as an outcome and are greatest between the first and second iterations of the assessment. Our primary outcome measure was a neuropsychological composite, rather than a single test, as endpoint, which provided greater statistical power for demonstrating a cognitive benefit (
29). A further implication of our study design is the importance of measuring an outcome repeatedly over the intervention period. This design might be a blueprint for testing procognitive drugs’ benefits in age-related cognitive decline. While our sample size was determined on the basis of a medium effect size of the vortioxetine–placebo difference for change in Toolbox Fluid Cognition Composite score, at two of the three outcome time points (weeks 4 and 26) we observed a small effect size (Cohen’s d values, 0.2–0.3). In randomized controlled trials in age-related cognitive decline, it may be appropriate to expect a small effect size for a procognitive intervention, so future studies may need larger sample sizes (150 or more per group) to be adequately powered.
Functional cognition, as measured by the UPSA, improved in both groups. It is unknown whether this increase in scores represented a true improvement in functional cognitive ability attributable to 26 weeks of cognitive training, or a practice effect. We found no significant interaction effects between the vortioxetine and placebo groups. One explanation of this is a true negative finding: drug augmentation of training by itself produces no transfer to function, and patients might need specific therapeutic help (known as cognitive remediation) to produce this transfer (
6,
8,
9,
22). There are other possible explanations: the study may have been underpowered to detect an effect on functional cognition, or the UPSA may have limitations in measuring functional cognition, including outdated tests of functional abilities not used by most contemporary populations (such as using 411 to call directory assistance or utilizing a bus schedule). Thus, further study is necessary to characterize the functional benefits of cognitive training when combined with vortioxetine.
Some limitations should be noted. This study was conducted at a single site. The study is positive in terms of the overall significant finding from the mixed-effects model, but the only significant individual time point was at week 12; thus, a confirmatory study is needed that could also determine the optimal duration of treatment. It may be that the true effect is largest after 3 months’ treatment, or that a larger study would show similar effects at all time points. Furthermore, at present we know little about the sensitivity to change or to intervention effects of the Toolbox Fluid Cognition Composite. The study also cannot address whether vortioxetine and cognitive training have an additive or interactive effect, such as vortioxetine driving beneficial plasticity such that the training is more efficient; a different study design, such as a factorial trial, would be needed to examine these possibilities. Also, the study leaves unclear the functional benefits of adding vortioxetine to cognitive training. Additionally, we studied the remediation of cognitive function, but we did not address the long-term implication; age-related cognitive decline is a chronic and, in many cases, progressive condition, with heightened risk for dementia. This raises the question: For what period of time might older adults need to continue taking vortioxetine and practicing cognitive training in order to continue to see benefits and slow cognitive decline? For clinical trials testing prevention of cognitive decline, large sample sizes and years-long per-protocol follow-up are needed (
30). Nevertheless, the present study suggests that a longer-term study is feasible and promising, given high adherence and retention in follow-up through 26 weeks. Other strengths were the maintenance of the blind and the good tolerability of vortioxetine, which are likely related, as a drug with significant side effects would be more difficult to mask. The high tolerability of vortioxetine at this dosage in older adults suggests that higher doses could be investigated to optimize the procognitive effects.
In summary, cognitive training in combination with vortioxetine is efficacious for improving global cognitive function in adults age 65 or older with age-related cognitive decline. This finding is important because computerized cognitive training programs offer a feasible and scalable combination with pharmaceutical treatment for older adults. Further research is needed to replicate this finding and to clarify its long-term and real-world benefits for the growing population of older adults.