Anxiety disorders are common impairing conditions (
1) that can endure after treatment in up to 50% of affected individuals (
2). Evidence suggests that the altered neurodevelopmental trajectory associated with anxiety may start as early as birth (
3–
5) and include an early-appearing excessive responsivity to unexpected or “deviant” stimuli. However, little is known about the specific neural systems underlying this excessive responsivity. In this study, we used task-based functional MRI (fMRI) to delineate the neonatal response to deviant stimuli and its relationship to maternal trait anxiety, a risk factor for problematic anxiety in offspring (
6,
7). An altered neonatal response to unexpected stimuli could signal increased risk for later anxiety, and the associated brain systems may provide targets for preventive interventions.
Previous research has related anxiety in children and adults to increased behavioral and physiological responses to environmental change (
8). Similar hyperresponsivity manifests in infants at risk for anxiety disorders (
3). Infants born to mothers with an anxiety disorder face a fivefold increased risk for anxiety disorders (
9). This increased risk exists along a continuum, as normative variation in maternal trait anxiety is associated with variation in offspring trait anxiety (
6), which in turn is related to later risk for anxiety disorders (
7). In nonclinical samples, higher antenatal maternal anxiety is associated with increased EEG evoked responses to deviant sounds in offspring during the neonatal period (
10) and at age 9 months (
11). Such increased EEG evoked responses are also associated with early childhood temperaments that predict risk for adult anxiety disorders (
3). Behavioral inhibition, an early-appearing temperament that includes increased reactivity to novel or deviant stimuli, is a potent risk factor for anxiety disorders (
3). Despite this behavioral and physiological evidence, the relevant brain regions remain unknown in infants.
The brain response to deviant stimuli and its relationship to anxiety are better understood in older children and adults than in infants. In adults, deviant stimuli engage numerous brain regions, including the left and right anterior insula, the dorsal anterior cingulate (dorsal ACC), the inferior frontal junction, and the ventrolateral prefrontal cortex (VLPFC) (
12). These regions derive from multiple “functional brain networks” that connect regions into distinct maps, such as the salience (
13), cingulo-opercular (
14), dorsal attention, and ventral attention networks (
15). Although the functional brain network architecture of the neonatal brain is incompletely understood, evidence indicates that network connectivity is lower in neonates, suggesting that networks are in a more immature form at that age (
16,
17). Anxiety disorders in older children and adults have been robustly associated with increased activity in regions within the salience, cingulo-opercular, and ventral attention networks in response to salient stimuli as well as salient task conditions such as making an error (
18). Beyond this increased salience response, recent meta-analyses indicate that anxiety and other psychiatric disorders are additionally associated with activity alterations in the anterior insula, dorsal ACC, VLPFC, subgenual anterior cingulate, and pregenual anterior cingulate during a range of cognitive (
19) and emotional (
20) tasks.
Examining brain function in neonates may illuminate risk markers that could be obscured in older samples as a result of compensatory adaptations. Previous research suggests that some of the neural correlates of pediatric anxiety disorders represent adaptive changes, such as increased prefrontal regulatory processes (
21). Measuring brain activity differences during infancy may uncover “core” deficits that occur before these secondary changes. Uncovering these early risk profiles is important given the prevalence (
22) and clinical impact (
23) of anxiety. Moreover, children with anxiety disorders face elevated risks in adulthood for many other disorders, including depression (
24), substance use disorders (
25), eating disorders (
26), and bipolar disorder (
27). Therefore, uncovering early risk profiles could inform attempts to identify mechanism-based targets that reduce the burden from many mental disorders.
To address these issues, we used fMRI to measure brain activity evoked by deviant auditory stimuli in 45 sleeping neonates. Following a commonly used strategy, we assessed the “oddball” response by playing loud white-noise bursts at irregular intervals in the context of a repeating sequence of background sounds (
12); in this case, the ongoing fMRI scanner sounds provide the background. Previous work has established the feasibility and utility of measuring fMRI responses to sounds in neonates (
28–
30). In addition, in a subset of 41 neonates, we related variation in the evoked response to normative variation in maternal trait anxiety, thereby modeling risk associated with mothers’ stable tendencies to experience anxiety in various situations (
31), a known familial risk factor (
6). We focus on familial risk because it may be transmitted by genetic or environmental effects, through in utero exposure to mothers’ biological stress response (
32). In post hoc analyses, we additionally controlled for confounding influences from maternal depression, reported stress, and state anxiety.
Methods
Sample
This study was approved by the Human Studies Committees at Washington University, and informed consent was obtained from all adult participants (the mothers) as well as parents of all neonatal participants. We recruited participants from a separate study at Washington University: the Early Life Adversity, Biological Embedding, and Risk for Developmental Precursors of Mental Disorders (eLABE) study. Participants in eLABE were themselves recruited during the second or third trimester of pregnancy, and eLABE collected a battery of maternal assessments as well as structural MRI in neonates (average age, 21.4 days; range, 3 to 40 days). Note that the participants recruited for this study comprised a normative sample that was not enriched for psychiatric symptoms. Parents of infants born full term (≥36 weeks’ gestational age) were asked to participate in the present study, which involved a subsequent task-based fMRI visit. This session occurred on average 6.8 days (range, 0 to 18 days) after the structural scan. Additional recruitment details, comparison of participants in the present study relative to all of eLABE, and inclusion and exclusion criteria are provided in the online supplement.
Maternal and Family Assessments
We utilized questionnaires from eLABE to record psychiatric symptoms and demographic data. The primary measure of maternal anxiety was the trait scale from the State-Trait Anxiety Inventory (STAI), which provides a stable metric of enduring trait-like anxiety (
31). The STAI was completed by 41 women, including 34 who completed the measure within a 5-week period after delivery and seven who completed the measure within an 8-week period following the child’s first birthday. Results did not change substantively when analyses were restricted to mothers who had completed the STAI near delivery (see Figure S10 in the
online supplement). Two women who completed the STAI near delivery had missing items on the state subscale only, resulting in 41 women with trait scores and 39 women with state scores. Additional assessments of psychiatric and demographic variables are detailed in the
online supplement.
Imaging Data Acquisition and Preprocessing
Imaging was performed without sedating medications using a Siemens 3-T Prisma scanner and a 64-channel head coil. Details on the procedures, fMRI parameters, and preprocessing are provided in the online supplement. We acquired between one and 11 fMRI blood-oxygen-level-dependent (BOLD) scans, depending on how well the infant tolerated the scan. All BOLD scans were collected on the same day. The scans were 5.7 minutes in length for the first 37 infants and 6.7 minutes in length for the next eight infants.
During each BOLD run, we played white noise auditory stimuli lasting 400 ms at irregular intervals, using E-prime (Psychology Software Tools, Sharpsburg, Pa.) and played via the external speaker on the scanner. Infants wore MiniMuffs ear protectors (Natus, Pleasanton, Calif.) to attenuate sounds to safe levels. Each run began with 56 seconds of background scanner noise (no white noise pulses). Next, a total of 24 oddball auditory stimuli were presented at random intervals every 9 to 14 seconds; the first oddball was always presented exactly 56 seconds after the start of scanning. In the first 37 infants, scanning ended after the last oddball; in the last eight, an additional 56 seconds of background scanner noise was played. Estimates were not substantively affected by the addition of this time (see Figure S14 in the online supplement). The background scanner noise from the BOLD sequence was regular and did not vary throughout the scan. The task paradigm is illustrated in Figure S1 in the online supplement.
We censored frames with framewise displacement greater than 0.9 mm (in Talairach atlas space) to reduce motion artifact. This cutoff has been empirically demonstrated to be optimal for task-based fMRI (
33); we repeated the primary analyses using a cutoff of 0.2 mm and obtained similar results (see Figure S11 in the
online supplement). Task runs with fewer than 150 remaining frames after censoring were excluded from the analyses, resulting in a total of two runs eliminated from a single subject. We obtained a median of 33 minutes of data for each infant (see Figure S1), including a median of 31 minutes (SD=14) after censoring.
Statistical Analysis
Preprocessed BOLD data were analyzed with a general linear model (GLM) as implemented using in-house software. Details of modeling are presented in the
online supplement. The auditory response was modeled by using separate finite impulse response regressors (
34) for each of the 40 BOLD frames following white noise onset (40 frames × 0.8-second TR = 32 seconds modeled).
We computed a whole-brain repeated-measures analysis of variance (ANOVA) with time point (1–40 frames after stimulus onset) as a within-subject factor using all subjects (N=45). The main effect of time point in this analysis indicates voxels with significant activity changes in response to the stimulus. Next, we computed a repeated-measures ANOVA in the subset of participants for whom maternal trait anxiety data were available (N=41). Factors were time point (1–40 frames after the stimulus onset), maternal trait anxiety on the STAI, and time point-by-maternal anxiety interaction. Maternal anxiety was treated as a continuous variable, although the data for two participants with high maternal anxiety scores were winsorized (see the online supplement). Figure S2 in the online supplement provides a histogram of maternal trait anxiety scores. We performed additional supplemental statistical analyses relating neonatal brain activity to maternal trait anxiety based on median split (see Figures S7 and S8 in the online supplement) as well as maternal state anxiety (see Figure S9 in the online supplement).
Data were spatially smoothed using a 6-mm full width at half maximum Gaussian kernel. All results were corrected for multiple comparisons to achieve a whole-brain cluster-wise error rate of p<0.01. Details on correction for multiple comparisons, derivation of regions of interest and time courses from statistical maps, network characterization, assessments of movement during scans, control for confounding variables, and tests to determine how the neural responses varied over the course of each run are provided in the online supplement.
Results
The sample’s demographic characteristics are summarized in
Table 1, and zero-order relations among variables are listed in Table S2 in the
online supplement. Maternal trait anxiety overall was in the normative range (STAI trait score, mean=31.2, SD=7.5, range=20–53) and was unrelated to socioeconomic status, infant sex, or gestational age. Based on a score ≥40 on the STAI state subscale (
35), 10.3% had clinically significant anxiety. As expected, maternal trait anxiety was significantly related to maternal state anxiety as measured with the STAI state subscale (r=0.71, p<0.001), stress as measured with the Perceived Stress Scale (r=0.60, p<0.001), and depression as measured with the Edinburgh Postnatal Depression Scale (r=0.50, p=0.001). Head movement overall was low (framewise displacement after censoring, mean=0.12 mm, SD=0.13) and was no more likely to occur at the onset of the sound relative to other times in the scan (F=1.12, df=39, 1716, p=0.28).
Neonatal Brain Response to Deviant Auditory Stimuli
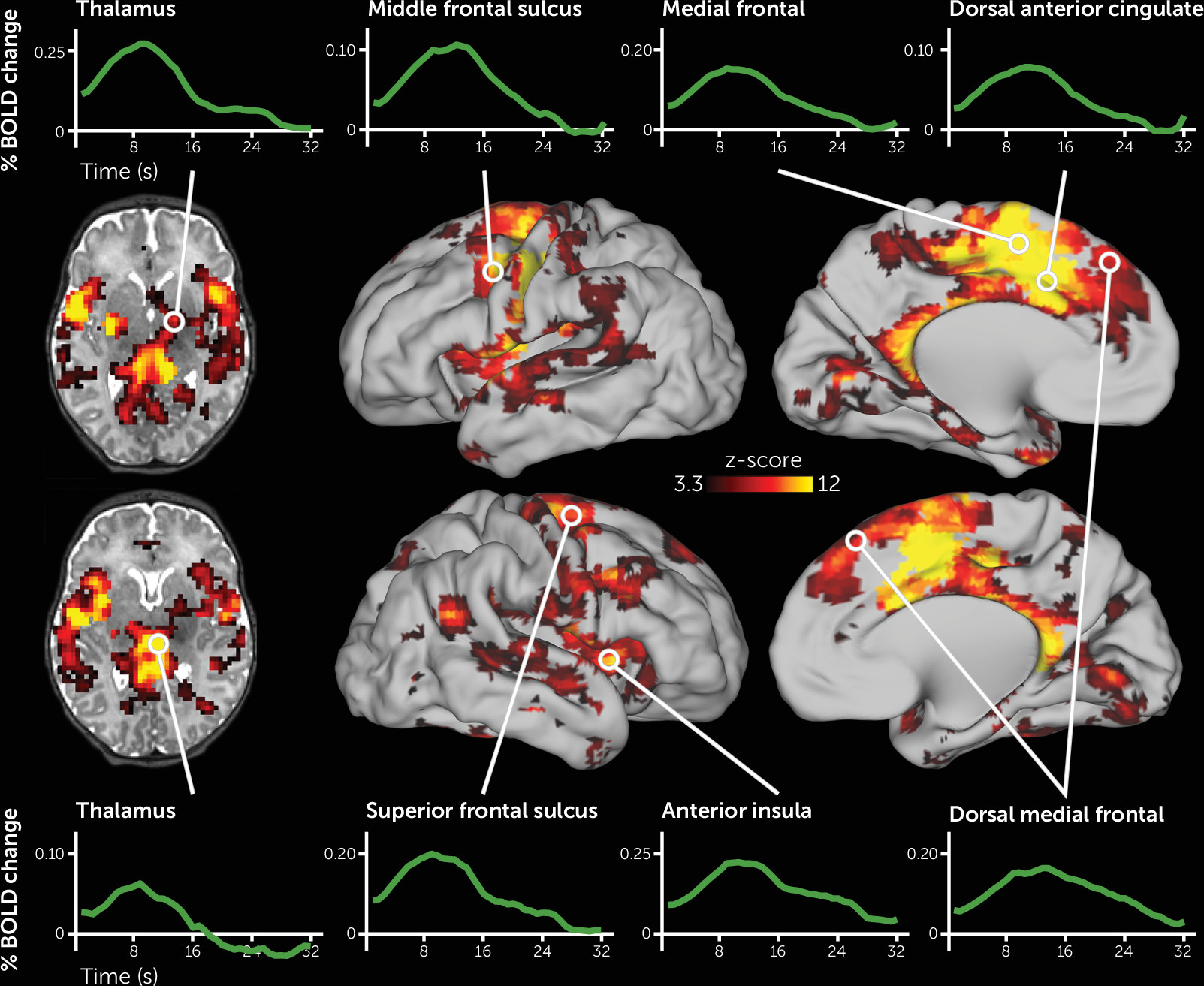
As depicted in
Figure 1, the deviant auditory stimuli elicited activity across a large portion of the neonatal brain. Specific regions included those that respond to auditory stimuli, including the thalamus, putamen, and auditory cortex. In addition, activity increased in regions of cortex that respond to deviant stimuli in adults, including the left and right dorsal ACC, anterior insula, and several discrete regions along the precentral gyrus, as well as in the right superior temporal sulcus extending posteriorly into the temporal-parietal junction (for a complete list of regions, see Table S3 in the
online supplement). Somewhat unexpectedly, the left motor cortex also demonstrated robust activity increases. All activity changes were highly statistically significant, and the shape of the fMRI response across these regions resembled fMRI responses to stimuli in older samples (see
Figure 1). Of the 177 regions demonstrating significant activity changes, 176 remained significant when we controlled for residual head motion (see Table S3 in the
online supplement). In most brain regions, neural activity changes were highest in magnitude for stimuli that were presented early in each fMRI run, intermediate for stimuli presented in the middle of each run, and lowest for auditory stimuli that were presented late in each run (see Figure S3).
To examine similarity with adults, we overlaid the neonatal brain response with results from a published meta-analysis of the response to deviant stimuli in adults (see Figure S4 in the
online supplement) (
12). Nearly all brain regions that demonstrated a response to deviant stimuli in adults also responded to deviant stimuli in the neonates. To probe how the neonatal brain functional architecture relates to the adult architecture, we registered adult network definitions (
36) to the neonatal brain (see Figure S5 in the
online supplement). While infant brain organization may differ from that of adults, we used adult network definitions because the organization of the neonatal brain is less well understood. For each adult-defined cortical network, we quantified the percentage of cortical surface area within that network that responded to the deviant stimuli in neonates (see
Figure 2). The adult-defined salience, cingulo-opercular, and ventral attention networks were among the networks with the highest percentage of surface area responsive to the stimuli in neonates.
Relation of Neural Response to Maternal Trait Anxiety
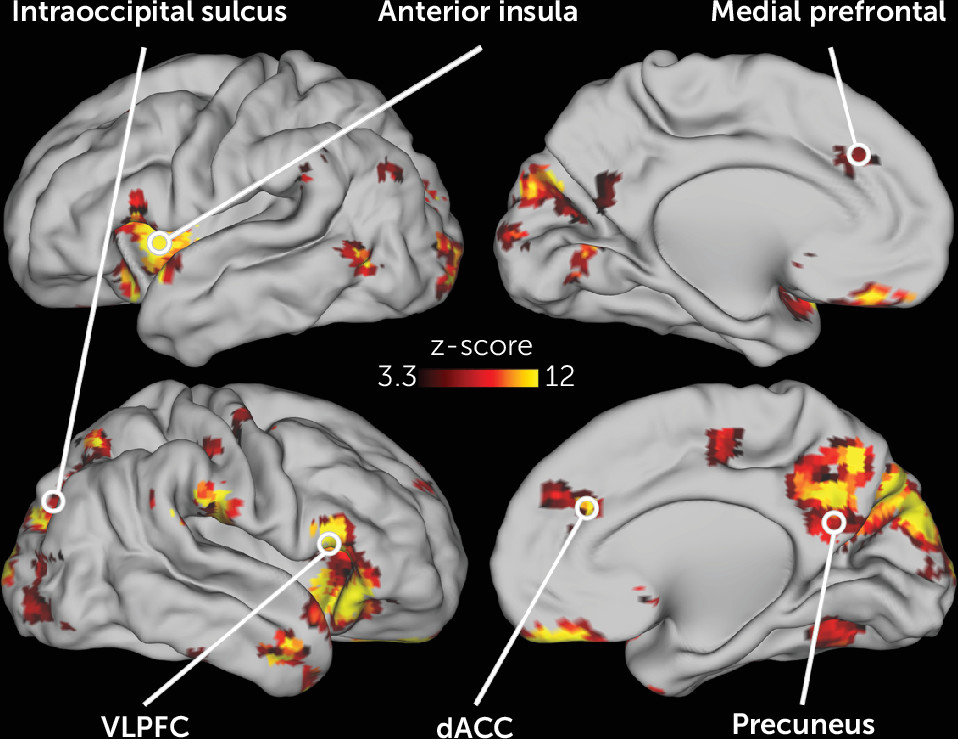
The activity in many different neonatal brain regions in response to deviant stimuli varied as a function of maternal trait anxiety (
Figure 3). Infants born to mothers with higher trait anxiety had higher activity in response to the deviant stimuli in the left and right anterior insula, dorsal ACC, subgenual anterior cingulate, and VLPFC (
Figure 4). In addition, in many regions in the occipital and posterior parietal cortex, activity following deviant stimuli was lower in neonates born to mothers with higher trait anxiety (
Figure 4). Of the 86 regions in which activity varied with maternal anxiety, 82 remained significant in post hoc sensitivity analyses that separately controlled for residual head motion, amount of retained data, state anxiety, depression, and stress (see Table S4 in the
online supplement).
We next characterized the adult network definitions of the neonatal brain regions in which activity varied with maternal trait anxiety (see Figure S6 and Table S4 in the online supplement). Brain regions with higher activity for neonates born to mothers with higher trait anxiety typically fell within the cingulo-opercular, salience, ventral attention, and anterior default mode networks. There were, however, some exceptions to this general pattern of results, with some cingulo-opercular, salience, ventral attention, and default mode network regions demonstrating lower activity in infants born to mothers with higher anxiety (see Table S4). Brain regions with lower activity for neonates born to mothers with higher trait anxiety tended to fall in the visual network and posterior portions of the dorsal attention and default mode networks.
Discussion
This study reveals that the neonatal brain exhibits a robust response to deviant auditory stimuli. The specific regions that respond are similar to those identified in adults in previous work and include the dorsal ACC, the anterior insula, the precentral gyrus, the right superior temporal sulcus, and the right temporal-parietal junction; in adults, these regions comprise primarily the salience, cingulo-opercular, and ventral attention networks. In a subset of regions, including portions of the VLPFC, anterior insula, dorsal ACC, and subgenual anterior cingulate, activity following deviant stimuli was higher in neonates born to mothers with higher relative to lower trait anxiety. In other regions, including nearby portions of the anterior insula, precuneus, and visual cortex, activity following deviant stimuli was lower in neonates born to mothers with higher relative to lower trait anxiety. Relations to maternal trait anxiety remained significant when we controlled for maternal state anxiety, depression, and reported stress.
Our results are consistent with other research on early childhood risk. This research suggests that neural stimulus-response properties near birth signal risk for cascades generating later-life anxiety and other psychiatric illnesses (
3–
5). Infants in the present study born to mothers with higher trait anxiety had increased neural responses in a subset of the brain regions that respond to deviant stimuli. Previous work suggests that early increased neural responsivity to novel stimuli continues through childhood and interacts with other altered processes, such as increased attention to threat or errors, to increase risk for an anxiety disorder later in life (
37). Of note, early childhood anxiety disorders are associated with additional psychiatric disorders later in life (
25–
27), which also have been linked to altered neural responses to deviant stimuli (
38–
40). Thus, the findings of the present study may inform the developmental neurobiology of other mental illnesses. Somewhat unexpectedly, infants born to mothers with higher trait anxiety additionally had lower activity in a separate set of brain regions; the functional significance of this lower activity is an important topic for future work.
Taken in the context of previous work, results from this study may inform the development of biomarkers for use in early risk stratification and as specific neural targets for preventive efforts. Maternal anxiety in this study largely varied across the normal range on the trait anxiety scale. Previous work indicates that such normative variation in maternal anxiety is associated with risk for psychiatric and neurocognitive outcomes in offspring during childhood (
41). This familial risk likely reflects impact from both genes and environmental factors, possibly transmitted though in utero exposure to maternal physiology. Results from the present study suggest that some of the increased familial risk for higher trait anxiety in neonates may manifest as alterations in basic stimulus-response properties. While similar altered stimulus-response associations have previously been observed using EEG, this study may clarify the specific brain regions and systems underlying risk. Regionally specific brain activity as measured with fMRI may inform specific targets that are more easily identified longitudinally across development relative to less regionally specific EEG-based measures. Such findings inform basic research in other species attempting to localize neural processes associated with risk.
In this study, we found that neonatal neural activity varies as a function of maternal trait anxiety in the same brain regions that have been linked to expression of anxiety in adults. A meta-analysis of 283 experiments that totaled over 10,000 participants identified a series of brain regions that robustly show differential brain activity in cognitive tasks between healthy control subjects and participants with major psychiatric illnesses, including anxiety disorders, depression, schizophrenia, and substance use disorders (
19). Regions identified by this meta-analysis included the left and right anterior cingulate and insular cortices and the right VLPFC. A more recent meta-analysis using the same approach but examining emotional rather than cognitive tasks additionally identified the subgenual and pregenual medial prefrontal cortices (
20). In the present study, we discovered that these same regions had higher activity in response to simple deviant sounds in sleeping neonates born to mothers with higher trait anxiety. This observation suggests either that previously reported results in children and adults pertain to alterations in basic stimulus-response properties rather than higher-level processes, or that variation in simple stimulus-response mechanisms at birth serves as the developmental foundation for disruption in higher-order processes later in life.
Delineating the neural architecture that responds to deviant auditory stimuli in neonates may also illuminate the fundamental neural building blocks of later, more complex processes relevant to risk for psychopathology. Previous work indicates that variation in lower-order brain processes in the first year of life is associated with variation in more complex cognitive brain processes later in childhood (
42). Brain regions that robustly responded to deviant stimuli in the present study, such as the anterior insula and dorsal ACC, are involved in more complex operations in adults, such as executive function and error monitoring (
14). Future longitudinal studies could evaluate whether variation in the response to deviant stimuli at birth predicts variation in other functions performed by these regions later in life, such as executive function.
This study should be considered in light of its limitations. The sample size was modest (N=45), although this was mitigated in part by a high amount of data per subject (∼30 minutes after frame censoring), which may provide for more reliable estimates of responses from each individual (
43). The sample included primarily mothers with nonclinical levels of anxiety; future studies can test whether these results extend to clinical samples. Maternal trait anxiety was highly correlated with other maternal symptoms, including depression and perceived stress. Although the results remained significant when we controlled for these factors, further work is required to disambiguate the impact of these various maternal symptoms on neonatal brain activity. Results in this study pertained to familial risk as assessed through maternal trait anxiety; further work is required to dissociate the impact of genetic and environment risk, such as exposure to circulating maternal stress hormones. We were unable to determine sleep stage in our participants, which may affect BOLD activity (
44). Finally, longitudinal studies are required to clarify the relation between the infant oddball response near birth and relations to observed temperament during infancy and clinical symptoms in later childhood.
In summary, this study reveals that the neonatal brain has a robust and specific response to deviant sounds and that the magnitude of neural activity in many brain regions is related to maternal trait anxiety. Furthermore, the specific brain regions that respond robustly to deviant stimuli, and the regional responses that vary with maternal trait anxiety, are the same regions that are implicated in the response to deviant stimuli and the pathophysiology of anxiety disorders in adults. These findings have significant implications for the developmental neurobiology of complex human behaviors and the origins of psychiatric disorders.
Acknowledgments
The authors thank Steven E. Petersen for comments on the manuscript and Joshua S. Shimony for reviewing the structural MRIs for neonatal injuries.