Psychostimulants, including methylphenidate-based and amphetamine-based agents, are first-line pharmacological treatments for attention deficit hyperactivity disorder (ADHD) (
1–
3), one of the most prevalent disorders of childhood (
4). While there are important differences in the actions of methylphenidate-based and amphetamine-based medications (
5), both medication classes appear to increase dopamine and norepinephrine action, thus affecting facets of cognition pertinent to ADHD symptomatology (
1,
5). Evidence from randomized double-blind placebo-controlled trials indicates that both subtypes of psychostimulants are efficacious relative to placebo in patients with ADHD (
1,
2). Although meta-analytic findings suggest that effect sizes for treatment response relative to placebo are larger for amphetamine-based (d=−1.02) than methylphenidate-based agents (d=−0.78), the latter are often better tolerated in children and adolescents with ADHD, and, among patients who respond well to at least one psychostimulant, the largest portion (48%) respond equally well to both medication types. In light of this greater tolerability, methylphenidate-based agents are the most commonly prescribed ADHD medication in this population (
1,
2).
Findings from in vivo neuroimaging studies converge on three sets of brain regions that are believed to be particularly relevant to the pathophysiology of ADHD and treatment response to psychostimulants (
6–
8). Several studies have investigated brain changes in response to acute doses of psychostimulant medication, reporting a normalization of cingulo-opercular and striato-thalamic hypoactivation and default mode deactivation during task-based functional MRI (fMRI) in patients with ADHD (
6,
8–
13). Abnormalities have also been reported in these networks at rest, although not without inconsistencies in the literature, perhaps in part due to methodological heterogeneity and small sample sizes (
14,
15). However, effects of psychostimulants on resting-state connectivity and metabolism within these networks have also been reported (
5,
16–
20; see reference
21 for a critical overview), as have effects on connectivity observed during task-based fMRI (
22).
The literature has two major limitations. First, symptom reduction in response to psychostimulants varies widely between patients, with an estimated 10%−30% not benefiting adequately from treatment, and the scientific literature provides only limited insights on the neural correlates of treatment responsivity (
1,
2). Although many neuroimaging studies have examined brain changes when patients are on versus off psychostimulant medication, little work has examined whether individual differences in treatment response are associated with differences in brain functioning, such as differences in brain functioning assessed while patients are off medication (
8,
12). Since psychostimulants are believed to act via a modulation of functioning within cingulo-opercular, striato-thalamic, and default mode regions, it is plausible that treatment response could be associated with functioning within these regions (
8). Second, ADHD is a developmental disorder, and the brain continues to develop throughout adolescence (
23–
25), with cross-sectional comparisons indicating differences in children and adolescents relative to adults with the disorder (
26), and longitudinal studies suggesting different neurodevelopmental trajectories in ADHD (
27). However, most studies have used cross-sectional designs and did not examine potential relationships between neurodevelopmental change in brain networks and treatment response to psychostimulants.
In light of previous work showing changes in functional connectivity with development, as well as pharmaco-fMRI work implicating these networks in the actions of psychostimulants (
8,
9), we examined whether resting-state connectivity within and between cingulo-opercular, striato-thalamic, and default mode networks would be associated with treatment response, as well as whether potential relationships changed with development. However, given that this is the first naturalistic study to examine possible associations between resting-state connectivity and long-term treatment response to psychostimulant medication, we did not form specific hypotheses regarding the direction of effects.
Results
Participant Characteristics and Treatment Response
The participants’ demographic and clinical characteristics are summarized in
Table 1. There was a higher proportion of females in the typically developing group (61/142, 42.96%) compared with the ADHD group (28/110, 25.45%) (χ
2=8.31, p=0.004), and sex was controlled for in all analyses. Patients were receiving methylphenidate-based medications during 132 observations (67.34%; mean dosage, 0.86 mg/kg per day of methylphenidate equivalents, range, 0.14–2.5) and amphetamine-based medications during 64 observations (32.65%; mean dosage, 0.82 mg/kg per day of methylphenidate equivalents, range, 0.23–2.14). A minority of participants switched between methylphenidate and amphetamine-based agents during the course of the study (N=7, 6.36%), and 69 participants (62.72%) always received methylphenidate and 34 (30.9%) always received amphetamine-based medications. Details on ADHD presentations are provided in the
online supplement.
Treatment response remained stable with age (B=−0.009, t=−1.14, p=0.26, 95% CI=−0.03, 0.007) and was not associated with psychostimulant dosage (B=0.04, t=0.8, p=0.4, 95% CI=−0.05, 0.1), sex (B=0.01, t=0.27, p=0.8, 95% CI=−0.09, 0.1), off-medication symptoms (B=0.0008, t=0.13, p=0.85, 95% CI=−0.01, 0.01), or psychostimulant class (methylphenidate or amphetamine-based; B=0.02, t=0.45, p=0.65, 95% CI=−0.07, 0.1). Off-medication symptoms declined with age (B=−0.53, t=−6.3, p<0.001, 95% CI=−0.7, −0.3). Psychostimulant methylphenidate equivalent dosage was not associated with age (B=−0.02, t=−1.56, p=0.12, 95% CI=−0.04, 0.006), psychostimulant class (methylphenidate versus amphetamine; B=0.05, t=0.62, p=0.5, 95% CI=−0.1, 0.2), or off-medication symptoms (B=0.005, t=0.49, p=0.6, 95% CI=−0.02, 0.02).
Owing to the accelerated design, some participants had more scans than others available at the time of analysis (e.g., because of having started the study earlier). However, patients with fewer than three scans did not differ from those with three or more scans with regard to treatment response (B=0.02, t=0.45, p=0.7, 95% CI=−0.09, 0.1), off-medication symptoms (B=0.02, t=0.28, p=0.79, 95% CI=−1.5, 1.9), in-scanner motion (B=−0.05, t=−1.5, p=0.13, 95% CI=−0.1, 0.02), dosage (B=0.04, t=−0.36, p=0.73, 95% CI=−0.2, 0.3), or psychostimulant class (methylphenidate or amphetamine; χ2=0.06, p=0.8). The primary finding also remained significant when analyses were restricted to the first two or first three scans (see the online supplement). Thirteen of the 110 patients (11.81%) stopped taking psychostimulant medication permanently while part of the study. Four of them stopped treatment and were in remission (three or fewer off-medication symptoms); two patients, who were showing “worse” treatment responses (i.e., worse than the mean treatment response), also stopped medication. Finally, seven stopped medication even though they were showing “better” responses at their last assessment (i.e., better than the mean treatment response).
fMRI Results
Network results.
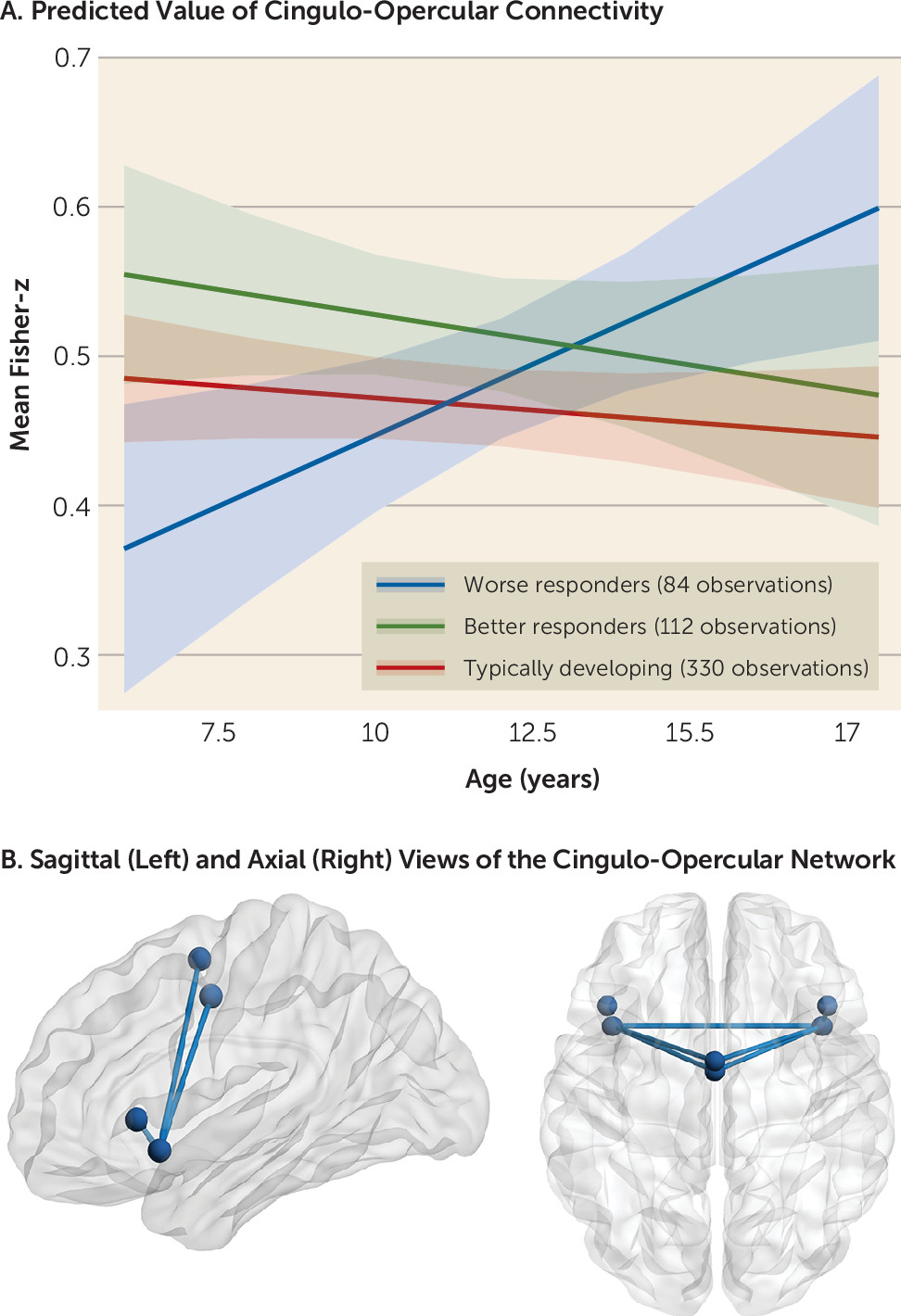
Model 1 showed that resting-state connectivity within the cingulo-opercular network was explained by a significant interaction between age and treatment response (B=−0.07, t=−3.4, adjusted p=0.006, 95% CI=−0.1, −0.03). No other within- or between-network metrics were significant even at an uncorrected threshold (p>0.1).
In model 2, a significant interaction between group and age on cingulo-opercular connectivity was found (F=4.86, df=2, 266, p=0.008). Specifically, age-related changes in connectivity that were associated with worse psychostimulant responses (i.e., a treatment response less than the mean; 84 observations from 63 participants; 25.4% female; mean age, 12.25 years) differed significantly from changes associated with better psychostimulant responses (i.e., a treatment response greater than the mean; 112 observations from 76 participants; 27.63% female; mean age, 11.33 years; B=−0.03, t=−2.79, p=0.006, 95% CI=−0.04, −0.008) and the typically developing control subjects (B=0.02, t=2.93, p=0.004, 95% CI=0.007, 0.04). In contrast, a better response to psychostimulant treatment was associated with age-related stability in cingulo-opercular connectivity that was similar to connectivity observed in the typically developing group (B=−0.003, t=−0.51, p=0.6, 95% CI=−0.02, 0.01).
Model 3 showed that worse responses to psychostimulant medication were associated with increased connectivity with increasing age (B=0.02, t=2.6, p=0.02, 95% CI=0.004, 0.04). Connectivity associated with better responses did not change with age (B=−0.007, t=−1.06, p=0.3, 95% CI=−0.02, 0.007), nor did connectivity change with age in the typically developing control subjects (B=−0.005, t=−1.49, p=0.14, 95% CI=−0.01, 0.001).
There were no significant main effects of treatment response or treatment response–by–age interactions for any other network metrics (all p values >0.1) (
Figure 1). Sensitivity analyses and robustness checks, including those related to in-scanner motion, number of scans available, psychostimulant subtypes, symptom subscales, and comorbid disorders, are provided in the
online supplement (see Table S2).
Edgewise results.
No findings survived correction for multiple comparisons at the level of individual region-of-interest–to–region-of-interest connections. Connections that were significant at an uncorrected p threshold of 0.05 are presented in Table S3 in the online supplement, and, in line with the network-level findings, include multiple connections between regions of the cingulo-opercular network.
Discussion
In this study, we examined whether treatment response to psychostimulant medication was associated with resting-state connectivity within a set of cingulo-opercular, striato-thalamic, and default mode networks implicated in pathophysiological models of ADHD and in the therapeutic actions of psychostimulants, as well as whether the associations changed with development. We found that psychostimulant response showed overall stability with age. At a neural level, we found that cingulo-opercular functioning was associated with worse treatment responses in a way that changed with development.
We assessed symptoms on and off medication at each time point, meaning that we could separate symptom change due to psychostimulants from age-related symptom reduction. We found that response to psychostimulants remained stable with age in our observational study. This finding is in line with a recent double-blind randomized placebo-controlled treatment discontinuation trial in which methylphenidate discontinuation in children and adolescents who had undergone treatment for at least 2 years was associated with a worsening of ADHD symptoms, as compared with treatment continuation (
36). The findings are also in line with pharmacoepidemiological work showing long-term associations between psychostimulants and decreases in injuries, accidents, and possibly substance abuse (see reference
37 for a critical review of this literature). Therefore, while causal inferences cannot be made given our observational design, our findings here are consistent with previous reports of stability in the efficacy of psychostimulant treatment with increasing age in children and adolescents with ADHD.
With regard to the brain, we found an association between psychostimulant treatment response and functional connectivity within the cingulo-opercular network. Better treatment responses were associated with a stable level of connectivity between nodes of this network during childhood and adolescence, whereas worse responses were associated with increased connectivity with increasing age. Moreover, typically developing children and adolescents showed age-related stability in cingulo-opercular connectivity, which tracked closely with the trajectory associated with better responses to psychostimulants. These findings are interesting in light of the literature reporting a normative segregation of networks during adolescence involving increasing within-network and decreasing between-network connectivity with age (
23). Since network segregation has been proposed to be adaptive and associated with improvements in cognitive functioning with age, the present findings of increasing within-network cingulo-opercular connectivity only in the worse treatment responders might be deemed surprising (
23). However, findings from longitudinal research on the development of within-cingulo-opercular-network connectivity have been mixed, and the fact that cingulo-opercular connectivity, at least as defined here, did not increase with age in the typically developing control subjects indicates that this increase associated with worse responses is atypical (
38,
39). While the exact nature of the relationship between functional connectivity and cognitive and behavioral development is unknown, it is generally accepted that complex cognition and behaviors depend on a finely balanced interplay between multiple interconnected networks (
23,
24). Consequently, the functioning of a given network is proposed to be highly dependent on the inputs and constraints provided by patterns of connectivity within and between other closely interdependent networks (
23). Therefore, connectivity patterns associated with worse treatment responses might be interpreted as developmentally atypical and potentially out of line with ongoing structural, functional, and neurochemical development in other brain regions and networks, as well as with ongoing age-related changes in behavioral maturation and environmental demands (
24).
That associations with treatment response were observed within the cingulo-opercular network is interesting in light of randomized placebo-controlled fMRI studies of acute methylphenidate effects in medication-naive boys with ADHD showing a normalization of functioning in this network (
10,
11,
13,
22,
40), as well as improvements in task performance (
11,
13,
40), with methylphenidate relative to placebo. Normalizing activation within the cingulo-opercular network therefore appears to be an important candidate mechanism of the acute effects of psychostimulant medication (
6,
8,
11). Few studies have examined whether the pattern or degree of acute neural changes associated with taking psychostimulants depends on neural functioning as assessed when off medication (
12). However, based on the present findings, the hypothesis emerges that psychostimulants may be better able to normalize recruitment within cingulo-opercular regions during cognitive demands when resting-state connectivity is already more in line with typical development. Alternatively, patients with already typical functioning in a network that undergoes modulation by psychostimulants may experience “better than typical” connectivity when medicated, which might allow them to compensate for their ADHD symptoms. No associations with treatment response were observed involving connectivity of the striato-thalamic and default mode networks, despite previous work showing changes in these networks in response to acute doses of psychostimulants (
9,
10,
12,
13). However, in this study, participants underwent scanning during the temporary cessation of psychostimulant medication, and it is likely that treatment response also depends on changes in neural functioning that are induced by psychostimulant medication (
12).
This study informs the understanding of the neural correlates of response to psychostimulants in a naturalistic setting. The findings suggest that characterization of developing functional connectivity may help us understand differences in psychostimulant responsivity. However, the study does not inform clinical decisions about the choice of psychostimulants, nor does it provide predictors of treatment response over time. Clinical translation of the findings depends not only on independent replication but also on the ability to characterize developing functional connectivity within a much shorter, clinically useful time frame than was used in this study. Furthermore, the costs of fMRI data need to be weighed against any gain in predictive power, particularly as psychostimulants act quickly, and adverse effects usually cease when the medication is stopped (
1,
3). Finally, given the observational design, we cannot draw any conclusions about the causal direction of the relationship between treatment response and developing functional connectivity. Therefore, we argue that rather than setting the stage for use of fMRI in clinical decision making in the near future, the contribution of the present study is to further our understanding of potential biological processes associated with differences in treatment response to psychostimulant medication, which has been limited.
Further limitations of the present work include the fact that the data were collected as part of a naturalistic observational study, and therefore the sample was heterogeneous with regard to prescribed psychostimulant medications, duration of treatment, and other clinical and demographic variables. Relatedly, we included patients on methylphenidate and amphetamine-based agents, which have partly distinct mechanisms of action (
5). However, we found that the relationship between cingulo-opercular connectivity, age, and treatment response did not vary significantly with psychostimulant class (see the
online supplement). An additional limitation is that since patients were receiving long-term psychostimulant treatment, this may imply that they had been receiving at least some benefit from the drug. Therefore, the findings may not generalize to very poor treatment responders, who are more likely to cease taking the medication (
2). In addition, ratings of ADHD symptoms were based on interviews with parents; therefore, they may have been biased by expectation effects on the part of parents, who were not blind to treatment. Moreover, we did not have objective data regarding treatment adherence and relied on parental report. Finally, as this analysis used data collected as part of a natural-history longitudinal cohort study, sample size was not determined according to a formal a priori power analysis. However, observed power estimates indicated that the study had adequate power to detect medium to large effect sizes for the interaction of interest in this study, in line with the detected effects (see the
online supplement).
In summary, we provide the first longitudinal investigation of the relationship between brain connectivity and treatment response to psychostimulants in youths with ADHD. We report that worse responses to psychostimulant treatment may be tied to developmentally atypical functioning within a cingulo-opercular network that has been implicated in both ADHD and psychostimulant action. The findings therefore speak to a potential factor contributing to differences in response to psychostimulant medication.