Major depressive disorder is the leading cause of disability worldwide (
1,
2), and approximately 50% of patients meet criteria for treatment-resistant depression (
3). Repetitive transcranial magnetic stimulation (rTMS), a brain stimulation treatment approved by the U.S. Food and Drug Administration (FDA) for treatment-resistant depression (
4–
6), targets the left dorsolateral prefrontal cortex (DLPFC), a key area in neural circuitry underlying depressive symptoms that has been shown to be hypoactive in major depressive disorder (
7–
9). Contemporary FDA-approved protocols for stimulation of the left DLPFC are limited by the long duration of treatment course (6 weeks) (
10) and have been only modestly effective, inducing remission after 4–6 weeks of treatment in ∼17% of patients who have not shown response to three prior antidepressant treatments (
11).
We developed an accelerated, high-dose, patterned, functional connectivity MRI (fcMRI)–guided rTMS protocol aimed at optimizing the treatment of treatment-resistant depression using neuroscience-informed stimulation parameters (
12,
13). Our protocol, previously referred to as Stanford accelerated intelligent neuromodulation therapy (SAINT) and now entitled Stanford neuromodulation therapy (SNT), includes 1) an efficient form of rTMS, termed intermittent theta-burst stimulation (iTBS) (
14); 2) treatment with multiple iTBS sessions per day at optimally spaced intervals (
15–
18); 3) application of a higher overall pulse dose of stimulation (
19,
20); and 4) personalized targeting for application of the stimulation of the left DLPFC to subgenual anterior cingulate cortex (sgACC) circuit (
21–
26).
Open-label trials of our SNT protocol have shown a remission rate of ∼90%, even in treatment-resistant individuals (
12,
13), which is almost double the remission rate of ECT for treatment-resistant depression (∼48%), the current gold-standard treatment (
27). However, the efficacy of SNT has not been investigated in a randomized sham-controlled trial. In the present study, we investigated the antidepressant efficacy of SNT in comparison to an identical schedule of sham stimulation to determine the contribution of the placebo effect.
DISCUSSION
The aim of this study was to investigate the antidepressant efficacy of SNT, an accelerated, high-dose, patterned, fcMRI-guided iTBS protocol, for treatment-resistant depression. Participants tolerated the treatment well; all participants completed the 5-day treatment course, and the side effect profile was similar to that of conventional daily rTMS, with a greater incidence of headache in the active SNT group compared with the sham treatment group. Headache is one of the most common side effects with TMS treatment protocols, so this was an anticipated side effect; the incidence of headache in our active treatment group (57%) is similar to that reported for the standard FDA-approved iTBS protocol for treatment-resistant depression (65%) (
10).
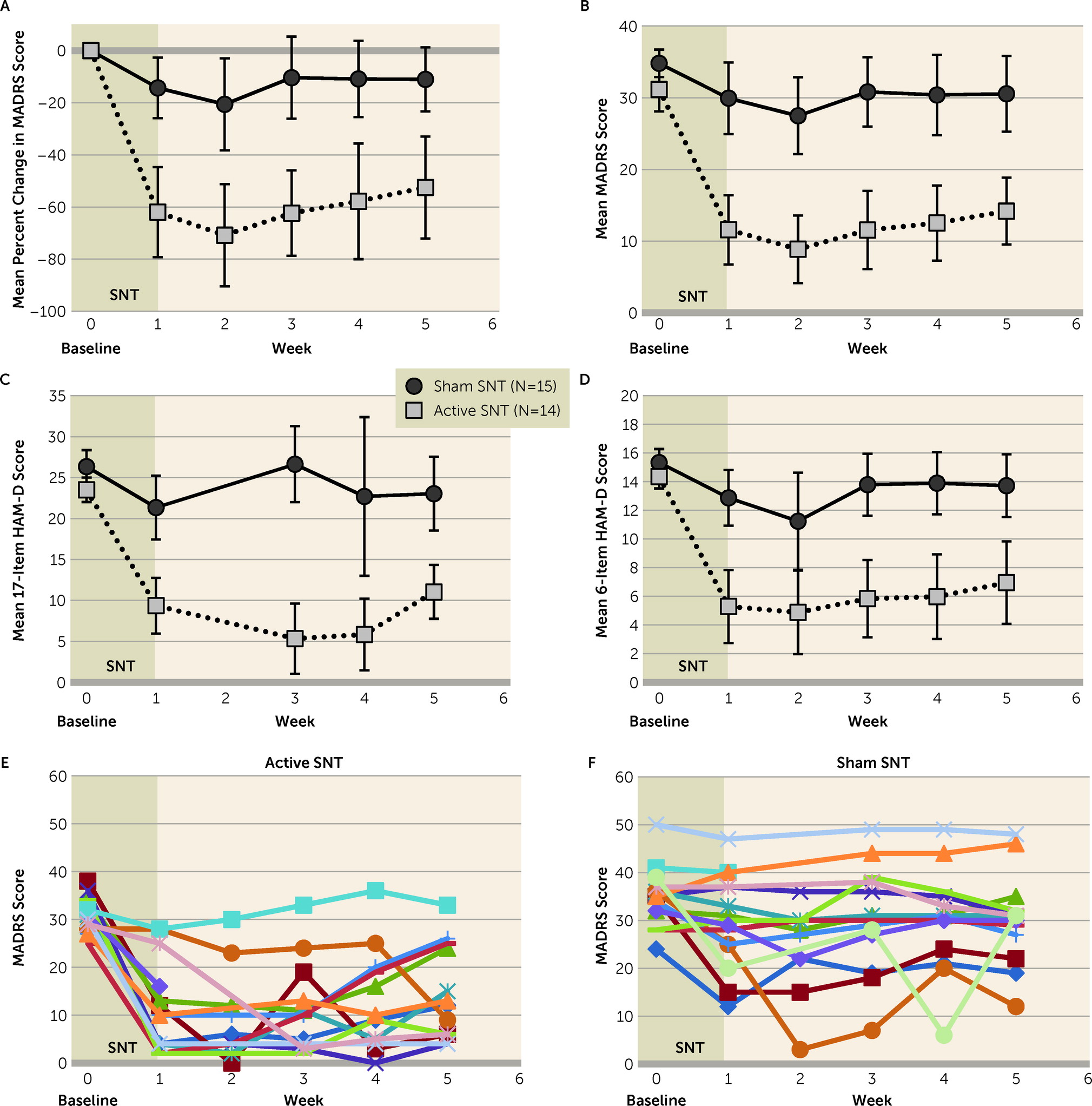
We observed a large antidepressant effect of SNT. After 5 days of treatment, 79% of participants in the active SNT group (11 of 14 participants) achieved remission from their depressive episodes at some point during the 4-week follow-up, compared with 13% (two of 15 participants) in the sham treatment group. The short treatment course and the high antidepressant efficacy of SNT present an opportunity to treat patients in emergency or inpatient settings where rapid-acting treatments are needed.
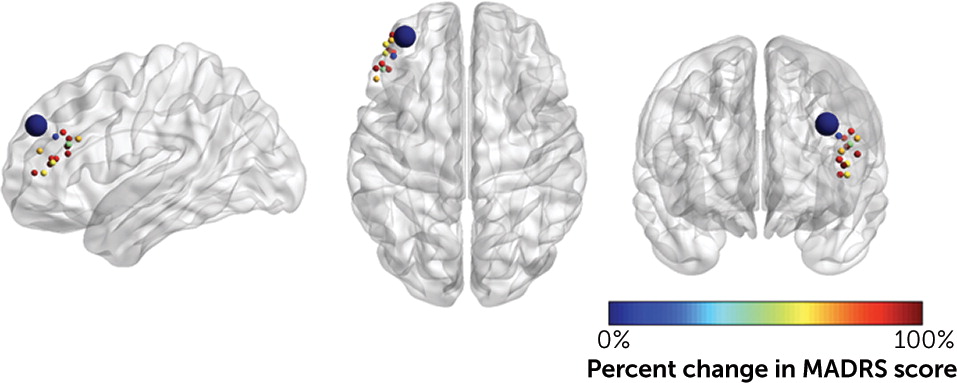
The large effect size of SNT may be due to several factors. The SNT protocol was designed from neuroscience-informed stimulation parameters and was selected to optimally modulate the targeted neural circuitry. First, fcMRI-guided targeting was used to stimulate the region of the left DLPFC most anticorrelated with the sgACC in each individual (
21–
26). Second, stimulation sessions were delivered hourly, because intersession intervals between 50 and 90 minutes have been shown to produce a cumulative effect on synaptic strengthening. In contrast, sessions with intersession intervals of 40 minutes or less do not produce a cumulative effect (
15–
18). Third, we delivered stimulation sessions at an intensity of 90% of resting motor threshold (rMT), adjusted for individual differences in cortical depth at the target, rather than the FDA-approved intensity of 120% of rMT, because iTBS sessions of 1,800 pulses delivered at 90% of rMT may be more effective in inducing changes in cortical excitability (
18) and the resultant functional connectivity changes (
44). Fourth, 1,800 pulses per iTBS session were delivered rather than the typical 600 pulses per session, as 1,800 pulses of iTBS at 90% of rMT has been shown to produce greater changes in cortical excitability (
18). Additionally, a blinded iTBS trial using sessions of 1,800 pulses produced effective antidepressant responses in only 2 weeks (
30), which is faster than standard FDA-approved iTBS protocols using sessions of 600 pulses (
10). Finally, the SNT protocol was designed to deliver a higher overall pulse dose than standard iTBS protocols (90,000 versus 18,000 pulses), because higher pulse doses are associated with greater antidepressant efficacy (
19,
20). SNT parameter choices were discussed in detail in our previous publications (
12,
13).
The large antidepressant effect size of SNT was observed despite the high severity of depressive symptoms and the level of treatment resistance in our participant sample as well as calculation at 1-month follow-up. Greater severity of depressive symptoms (
45,
46) and higher levels of treatment resistance (
4,
6,
47) have both been associated with poorer responses to rTMS. The participants in our study had an average of five previous adequate antidepressant medication trials; conventional rTMS protocols have been found to induce remission in only 17% of individuals who have shown no response to three prior antidepressant treatments (
11). Additionally, 62% of our participants had recurrent depressive episodes rather than one continuous episode, which has also been shown to negatively predict outcome following rTMS treatment (
46). The high level of treatment resistance in our sample may also account for the relatively low remission rate observed in our sham treatment group (13%) in comparison to remission rates typically reported for sham treatment in rTMS studies (
48,
49); a previous sham-controlled iTBS study showed that individuals with moderate to severe treatment resistance exhibited minimal response to sham treatment (
30).
In this report, we included response and remission rates for the entire 4-week follow-up period. This is a departure from the more common single-time-point criterion for response or remission used in other iTBS clinical trials for depression (
10). We believe this approach to defining dichotomous outcomes is appropriate based on the use of SNT as a probe of the underlying neurocircuitry of depressive symptoms and the intended future clinical approach. These high overall response and remission rates provide further evidence to support that dysfunction in the left DLPFC-sgACC network is the predominant neural basis of depressive symptoms (
21,
22,
24,
50). This study expands on the literature by demonstrating that when this circuitry is targeted using an optimized stimulation dose and pattern, antidepressant responses will be induced in the large majority of individuals (85.7% in the present study) for a period of time. For unknown reasons, participants display different response trajectories following a course of SNT (see
Figure 2E), which has previously been reported for rTMS and iTBS (
43). SNT is still being developed for use as a therapeutic tool. We propose a treatment model in which SNT is used to achieve rapid remission from depression and is then followed by a less intensive maintenance treatment that can be of any effective and acceptable modality—medication, psychotherapy, brain stimulation, and other treatments. Under this model of care, all patients who enter remission in the month after treatment would be able to transition to a maintenance treatment. Therefore, it is appropriate to calculate response and remission rates by including all participants who met these criteria at any week during the 4-week follow-up.
This study has limitations. First, the sample size was small, as the trial was ended at the planned interim analysis because of superiority of the active treatment with a large effect size; yet, the sample size is similar to those of other clinical trials in patients with severe treatment-resistant depression (
51,
52). Second, as with all clinical trials for major depressive disorder, our study relied on clinical assessments to measure improvement in depressive symptoms, as there are currently no validated biomarkers of depression remission. Third, thus far our SNT protocol has been tested at a single site, in a highly educated sample. Although this limits generalizability, the absence of a significant sham response in this treatment-resistant population indicates clear efficacy of active over sham stimulation for this patient population. Fourth, 45% of our participant sample had comorbid psychiatric diagnoses (see Table S4 in the
online supplement), which could have influenced the efficacy of the protocol. Comorbid anxiety in particular has been shown to reduce rTMS efficacy (
4,
53,
54), perhaps because of the need for an alternative treatment target for these patients (
55) or a higher incidence of benzodiazepine use in this population (
56,
57). However, the number of participants with comorbid psychiatric conditions was not significantly different between the active and sham treatment groups (see Table S4). Finally, given the large effect size in comparison to other TMS protocols in similar populations, we hypothesize that the differences between SNT and standard rTMS protocols lead to high antidepressant efficacy; however, SNT remains to be tested directly against another active protocol, and the unique aspects of the SNT protocol that account for improvements in efficacy over conventional iTBS remain to be identified. Studies will be needed that compare the efficacy of SNT parameters with and without fcMRI-guided targeting to determine the importance of this targeting method (
58).
In conclusion, SNT induced a significantly greater reduction in depressive symptoms than an identical course of sham stimulation after 5 days of treatment in a treatment-resistant sample. The short duration and high antidepressant efficacy of SNT presents an opportunity to treat patients in the emergency or inpatient settings, where a compressed time course is necessary.