Opioid analgesics have been increasingly prescribed worldwide, especially in North America (
1). Both Canada and the United States saw their prescription opioid consumption increase until 2010, then stabilize and decline following various control interventions (
2–
4). Among the up to 100 million North Americans annually exposed to prescription opioids, it is estimated that 5%–24% misuse them and 1%–9% develop an opioid use disorder (OUD) (
5,
6). These individuals represent 88% of people with OUD, which also includes heroin use disorder (
7). While safer prescription practices have been implemented to reduce opioid exposure and related harms (
8), highly potent illicit synthetic opioids (e.g., fentanyl) have recently proliferated, and they contributed to 82% of overdose deaths in Canada in 2020 (
9). The COVID-19 pandemic exacerbated this problem, increasing estimated opioid mortality by 34%–89% (
9,
10). Taken together, prescription and synthetic opioid (prescription-type) OUD (POUD) and related harms represent the third highest burden of disease attributable to substance use after tobacco and alcohol (
11). Prevention and treatment of OUD have become a public health priority.
The epidemic of overdoses and the large-scale harms related to prescription-type opioids have triggered many calls for a broad public health approach to address this crisis (
12–
14). Various multifaceted strategies have been proposed to tackle this complex problem: interventions to improve drug supply safety; sterile injection equipment provision; supervised drug consumption sites; naloxone distribution; testing and treatment of frequent comorbid health conditions (e.g., human immunodeficiency virus [HIV], hepatitis C virus [HCV]); and evidence-based psychosocial and pharmacological treatment for OUD (
14). One key component is the provision of opioid agonist therapy (OAT) for those who have developed OUD (
14,
15). This approach, as with most if not all of the aforementioned strategies, is unfortunately far from being systematically implemented and often is either inaccessible or is not adapted to the individuals who need it the most. Addressing barriers to treatment and the use of more flexible models of care for OAT represent a critical step toward a global effective approach to tackle the OUD and overdose crisis (
14).
The OAT options include buprenorphine (alone or combined with naloxone to prevent overdose [
16]), methadone, and, in some countries, extended-release morphine and other opioids (
15). While methadone has long been the standard of care in Canada, buprenorphine/naloxone has been the preferred treatment in many other countries, including the United States (
17,
18), and it is now the recommended first-line treatment in Canadian guidelines as well (
19). Methadone’s low therapeutic index (i.e., a small difference between therapeutic and toxic doses) motivates a strict witnessed consumption, especially during initiation, either in specialized clinics (e.g., in the United States) or in community-based pharmacies (e.g., in the United Kingdom, France, and Canada) (
19). It is only after a few months of supervised daily ingestion that patients may receive take-home methadone at their physician’s discretion (
19), which may demotivate patients (
19–
21). The superior safety profile of buprenorphine/naloxone offers the potential advantage of early flexible take-home dosing (
19), which could be more acceptable (
22) to people with OUD without necessarily hindering efficacy (
23,
24).
In the context of the ongoing COVID-19 pandemic and beyond, decreasing the requirements for in-person visits related to supervised ingestion could be critical to providing flexible, more acceptable, less costly, and better-adapted OAT models of care. Extant trials, however, have compared the efficacy of buprenorphine/naloxone with methadone when these pharmacotherapies are offered under similar strict supervision (
25). Additionally, while both treatments are efficacious in individuals with OUD (
25,
26), the evidence mainly stems from efficacy trials in patients primarily using heroin (
27–
30), who may differ from individuals with POUD in terms of the potency of the substances they consume, comorbidities, and risk behaviors (
15,
31). Overall, the quality of evidence to support flexible take-home OAT strategies remains low and insufficient to guide clinical practice for POUD (
32).
Therefore, randomized controlled trials are needed to compare more flexible models of care for POUD to existing approaches. In this pragmatic trial evaluating the effectiveness of buprenorphine/naloxone administered under a flexible take-home dosing regimen compared with standard and witnessed methadone treatment in Canadians with POUD (
33), we hypothesized that buprenorphine/naloxone would be noninferior to methadone in reducing opioid use.
Methods
Study Design
In this phase 4 pragmatic, open-label, noninferiority, two-arm parallel (
33) randomized controlled trial, we evaluated the effectiveness of buprenorphine/naloxone relative to methadone as OAT for individuals with POUD.
Participants
We included treatment-seeking participants ages 18–64 diagnosed with OUD, based on DSM-5 criteria, related to prescription-type opioids (licit or illicit, including fentanyl, and whether or not they have been prescribed) and requiring OAT. Participants were excluded if they had any unstable psychiatric or medical condition precluding safe participation, experienced pain requiring opioids, used heroin as the most frequent opioid in the past 30 days, were enrolled in OAT 30 days prior to screening, took medications that interact with the study medications, had a history of severe adverse reaction to the study medications, or had pending legal action preventing study completion. Women who were pregnant, breastfeeding, or planning to conceive were excluded. All participants were compensated up to $560 ($40 per visit) for their time when completing study visits.
Setting
This multicenter trial recruited participants in seven Canadian hospitals and community-based clinics: the Rapid Access Addiction Clinic (Vancouver), the Portland Hotel Society Medical Clinic (Vancouver), the Opioid Dependency Program Clinic (Calgary), the Centre for Addictions and Mental Health (Toronto), the Ontario Addiction Treatment Centre (Sudbury), the Centre Hospitalier de l’Université de Montréal (Montreal), and the Centre de Recherche et d’Aide pour Narcomanes (Montreal). Canadian opioid treatment programs allow patients to receive a prescription in a clinic or hospital and go to their local pharmacy for supervised or unsupervised OAT administration. Participants’ follow-up ended on July 29, 2020.
This study followed international and local ethical guidelines, including the Helsinki Declaration, the Good Clinical Practice guidelines from the International Council of Harmonization, the Tri-Council Policy Statement on Ethical Conduct for Research, and Health Canada Division 5 guidelines. Each clinical site’s ethics committee approved the study. The trial was registered at ClinicalTrials.gov (NCT03033732) prior to enrollment.
Randomization and Masking
Interested individuals first verbally consented to be prescreened before meeting the research staff for full eligibility assessment. After receiving a complete description of the study, eligible participants were quizzed on the study and signed a written consent form at the screening visit. They were then randomized in a 1:1 ratio to receive buprenorphine/naloxone or methadone. We used a stratified permuted block design with blocks of varying size, and an independent statistician created the computer-generated randomization sequence, stratified by site and lifetime heroin use. As this trial was open label, no masking was done, and both participants and clinicians knew the nature of the intervention. The research staff notified participants of their treatment assignment.
Procedures
Randomized participants met with a study physician to receive the assigned medication and discuss their treatment plan and induction procedures. Treatment initiation started within 14 days after randomization. All participants underwent supervised ingestion of their first dose either at the clinic or at the pharmacy. Study sites and community pharmacies also dispensed medications for the remainder of the intervention period. Participants attended follow-up visits every 2 weeks for 24 weeks, during which they provided urine samples and updated their demographic, drug use, and medical information. Participants were also optionally screened for HIV and HCV as per available guidelines. Women were tested monthly for pregnancy. As previously described (
33), all interventions followed national and provincial guidelines for the management of OUD together with Health Canada product monographs (
19,
34–
39).
In Canada, buprenorphine is available in combination with naloxone. Most participants initiated buprenorphine/naloxone treatment at a 4 mg/1 mg dose, with additional doses (up to 12 mg/3 mg) sublingually administered on the first day. Titration continued as needed during the following days, up to a maximum recommended daily dose of 24 mg/6 mg. Clinically stable participants could receive take-home doses at their physician’s discretion, with a suggestion of 1-week carries within 2 weeks after initiation and 2-week carries within 4 weeks after initiation, except if unsafe or clinically inappropriate (i.e., inability to safely store buprenorphine/naloxone with risk of theft, loss, or access by minors; significant diversion of buprenorphine/naloxone with reasonable expectations that restricting carries would decrease the risk of such diversion; current comorbid sedative or alcohol use disorder compromising the participant’s safety; and voluntary overdose involving buprenorphine and compromising participant’s safety).
Participants in the control group initiated methadone with a maximal oral dose of 30 mg on the first day. Dosages were gradually titrated (i.e., 5–10 mg/day every 4 or more days), with typical target dosages of 60–120 mg/day. After 2–3 months of supervised ingestion, stable participants could take home doses at their physician’s discretion according to all Canadian local guidelines.
Outcomes
Our primary outcome was opioid use, measured by the proportion of opioid-free urine drug screens during the 24 weeks, with missing values defined as positive. Urine drug screens from participants who discontinued their assigned treatment, switched to any other OAT, or attended a visit outside the ±7-day window were also considered positive. Urine specimens were analyzed using a Rapid Response Multi-Drug One Step Screen Test Panel and single test strips for both hydromorphone and 6-monoacetylmorphine. We tested for the presence of morphine, oxycodone, fentanyl, benzodiazepines, cocaine, amphetamine, methamphetamine, ∆-9-tetrahydrocannabinol, buprenorphine, methadone, and tramadol. We checked the tests’ validity with a commercially available adulterant test.
Retention in treatment, defined as the proportion of participants having both an active prescription and a positive urine drug screen result for the assigned OAT at week 24, was a secondary outcome. Participants switching OAT during the trial were not considered retained in the assigned treatment for this outcome, but retention on any OAT (i.e., buprenorphine/naloxone, methadone, diacetylmorphine, slow-release morphine, hydromorphone) at week 24 was also assessed and reported. Quality of life, an exploratory outcome, was assessed at baseline and every 4 weeks with the EuroQol-5D (
40), which includes a visual analogue scale (EQ-VAS).
Safety
We monitored all adverse events and serious adverse events from screening up to 30 days after study end. The study physicians graded all adverse events’ severity and relatedness with the study medication. An independent data safety and monitoring board examined participants’ safety data every 6 months.
Statistical Analysis
Power calculation.
We expected to measure an absolute mean difference of 7.5% for opioid-free urine drug screens between the methadone (75%) and the buprenorphine/naloxone (67.5%) arms together with a 25% standard deviation. Following an iterative consultation process with addiction specialists and researchers, the noninferiority margin was consensually set at 15%. Based on these assumptions, we performed a power calculation using an alpha level of 0.05 (one-sided), 80% power, and 1:1 allocation ratio (using R, version 3.3.1), resulting in 276 participants (138 per group).
Primary, secondary, and exploratory analyses.
The mean difference in opioid use between the buprenorphine/naloxone and methadone arms and its 95% lower confidence limit (a one-sided alpha of 0.05) were computed using an analysis of covariance and SAS, version 9.4 (SAS Institute, Cary, N.C.). The Brown-Forsythe test and observation of the residual plot verified the assumptions of variance homogeneity and data normality. A multiple logistic regression estimated the effect of buprenorphine/naloxone treatment on retention relative to methadone. The odds ratio measured treatment effect for the binary retention outcome. We performed modified intention-to-treat analyses on all outcomes. Following a software-related protocol deviation affecting the allocation table at three sites (N=40/272, 14.7%), we analyzed the data based on the treatment actually allocated at randomization (versus the one that was intended). A generalized linear mixed model evaluated the treatment effect, time effect, and treatment-by-time interaction effect on quality of life, with time points clustered within subjects. Within-person correlation was handled using a compound symmetry for the covariance matrix structure. All longitudinal assessments were included in this analysis. Results were adjusted for stratification variables (site and lifetime heroin use).
Adverse events were analyzed using the Medical Dictionary for Regulatory Activities (MedDRA) terms. The number and percentage of participants experiencing adverse events were tabulated by severity and relationship to treatment. The relative risk, confidence interval, and p value of experiencing categories of adverse events comparing groups were computed. The hazard ratio, 95% confidence interval, and p value of experiencing any drug-related adverse event comparing groups were computed with the Andersen-Gill proportional hazards models stratified by site and robust variance estimates adjusting for the correlation of events within participants.
Handling of missing data.
Analyses were based on collected data. Aside from the single imputation for missing urine drug screens that were considered positive, there was no other imputation for missing values. Given the COVID-19 pandemic, follow-up assessments were conducted by telephone instead of in-person starting on March 16, 2020. No urine drug screens could be performed, and the values were considered missing completely at random.
Sensitivity and subgroup analyses.
First, we conducted four sensitivity per-protocol analyses for the primary outcome: 1) including only retained participants, 2) including only participants who initiated the assigned treatment, 3) excluding participants with missing data, and 4) excluding the 12 participants with risk of false positive urine drug screens (some urine drug screen cups and hydromorphone strips cross-reacted with methadone). Second, we performed modified complete-case analyses for all outcomes, 1) excluding 10 participants with missing values due to COVID-19 and/or 2) removing missing data due to COVID-19. Third, we analyzed (exploratory post hoc) the main outcome by subgroups for fentanyl use (i.e., urine drug screen positive for fentanyl at least once during the study, versus none). Fourth, we performed an exploratory post hoc modified intention-to-treat analysis for opioid use on the first and the last 12 weeks of follow-up. Lastly, we performed sensitivity analyses on the retention outcome using alternative definitions: 1) having only a prescription for the assigned OAT at week 24 (a priori) and 2) having a prescription and a positive urine drug screen for any OAT (methadone, buprenorphine-naloxone, long-acting morphine, or any other opioids used as an OAT) at week 24 (post hoc).
Results
Recruitment spanned the period between October 2, 2017, and March 23, 2020. The COVID-19 pandemic led the study team (after consulting the data safety and monitoring board) to stop recruitment with 272 randomized participants (of the 276 initially planned), with negligible impact on statistical power.
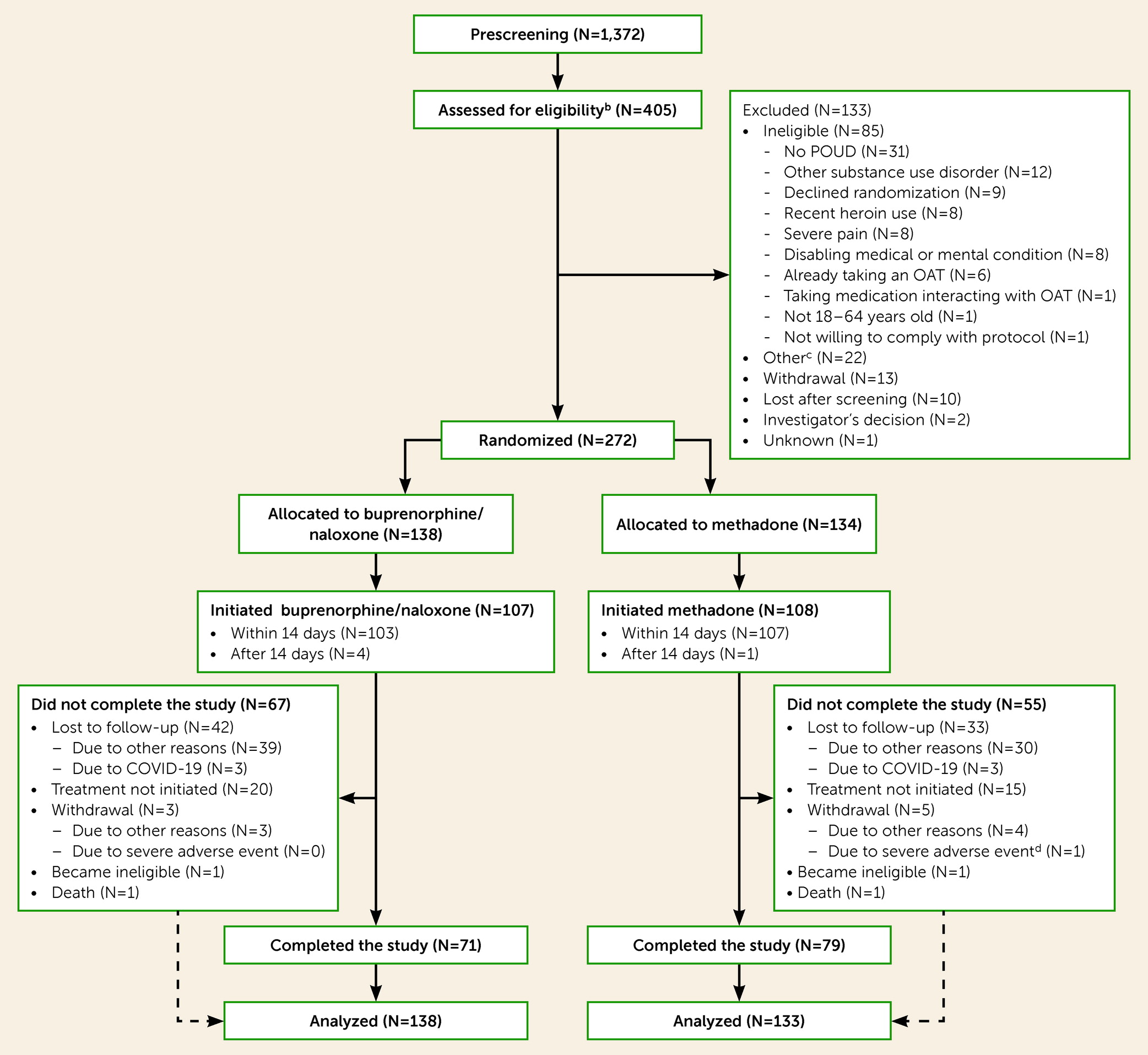
Figure 1 shows the CONSORT flow diagram of participants.
Table 1 summarizes the participants’ sociodemographic characteristics. One participant in the methadone arm died soon after initiating treatment; 271 participants were analyzed. Follow-up lasted on average 101.4 days (SD=76.3) and 111.7 days (SD=74.9) for the buprenorphine/naloxone and methadone groups, respectively. Among retained participants, the mean maximum dose taken during the study was 20.3 mg (SD=7.4) for buprenorphine (N=32) and 81.8 mg (SD=37.3) for methadone (N=45). The proportion of participants who switched to any other OAT was 31/138 (22.5%) in the buprenorphine/naloxone group and 16/134 (11.9%) in the methadone group. The proportion of participants initiating treatment who received take-home doses was 73.8% (76/103) in the buprenorphine/naloxone group and 32.1% (34/106) in the methadone group. The average time between treatment initiation and the first take-home dose was 12.7 days (SD=20.3) for the buprenorphine/naloxone group and 85.2 days (SD=39.8) for the methadone group. Participants had, on average, 4.1 take-home doses of buprenorphine/naloxone (SD=3.2) or 2.1 take-home doses of methadone (SD=1.8) when take-home doses were first prescribed. The mean maximum number of consecutive days of take-home doses was 13.1 days (SD=12.3) for the buprenorphine/naloxone and 4.9 days (SD=5.1) for the methadone group.
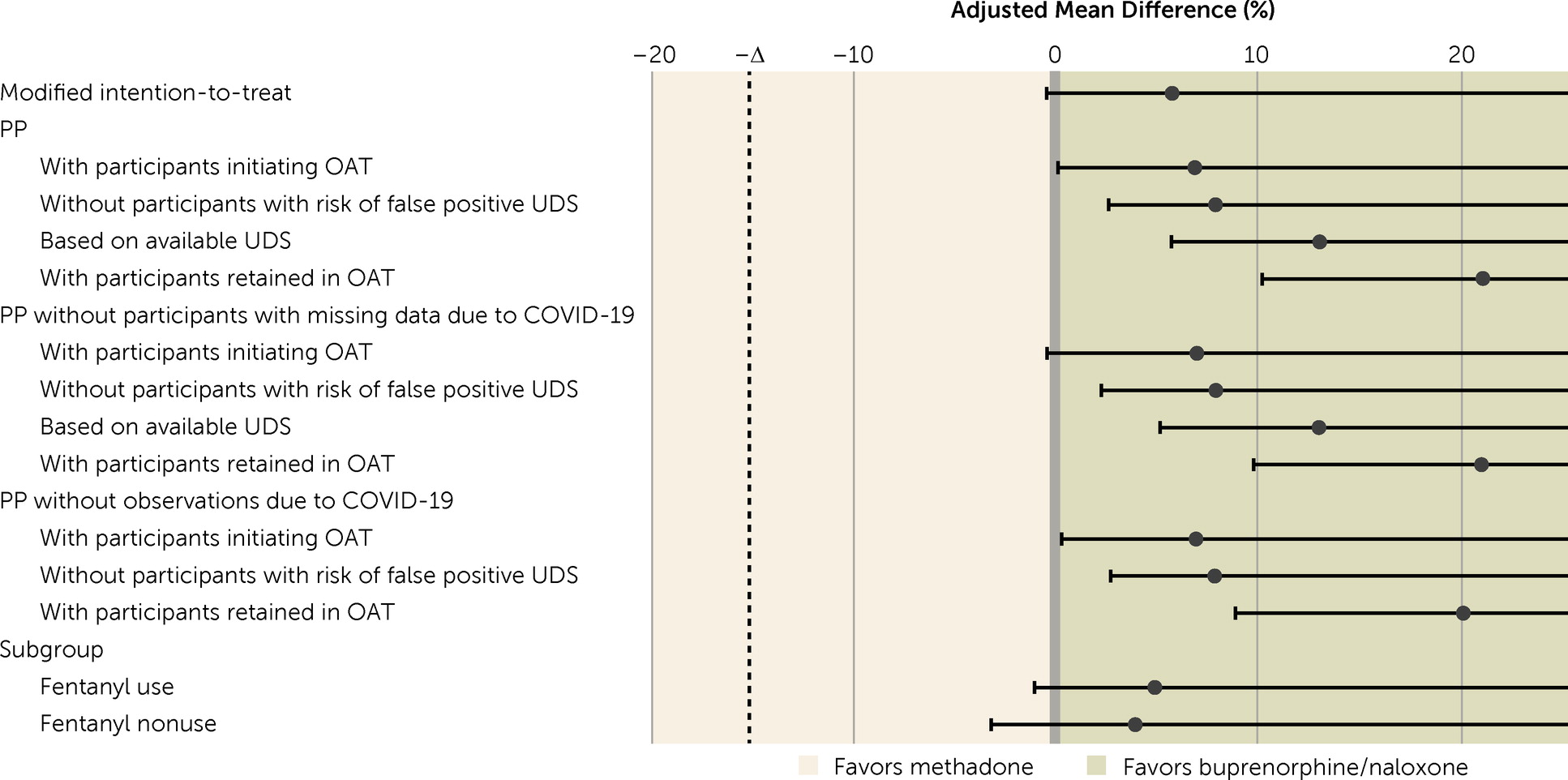
The mean proportion of opioid-free urine drug screens was 24.0% (SD=34.4) in the buprenorphine/naloxone group and 18.5% (SD=30.5) in the methadone group. The adjusted mean difference in opioid-free urine drug screens was 5.6% (95% CI=−0.3, +∞, p=0.040) and demonstrated noninferiority in all modified intention-to-treat and per-protocol analyses (
Figure 2,
Table 2). Post hoc analysis showed that the adjusted mean difference was 8.7% (95% CI=3.0, +∞, p=0.0065) in the first 12 weeks of treatment and decreased to 2.4% in the last 12 weeks (95% CI=−3.3, +∞, p=0.24) (see Table S1 in the
online supplement). The number of collected urine drug screens did not vary by treatment group (buprenorphine/naloxone group, 817/1,655, 49.4%; methadone group, 900/1,596, 56.4%).
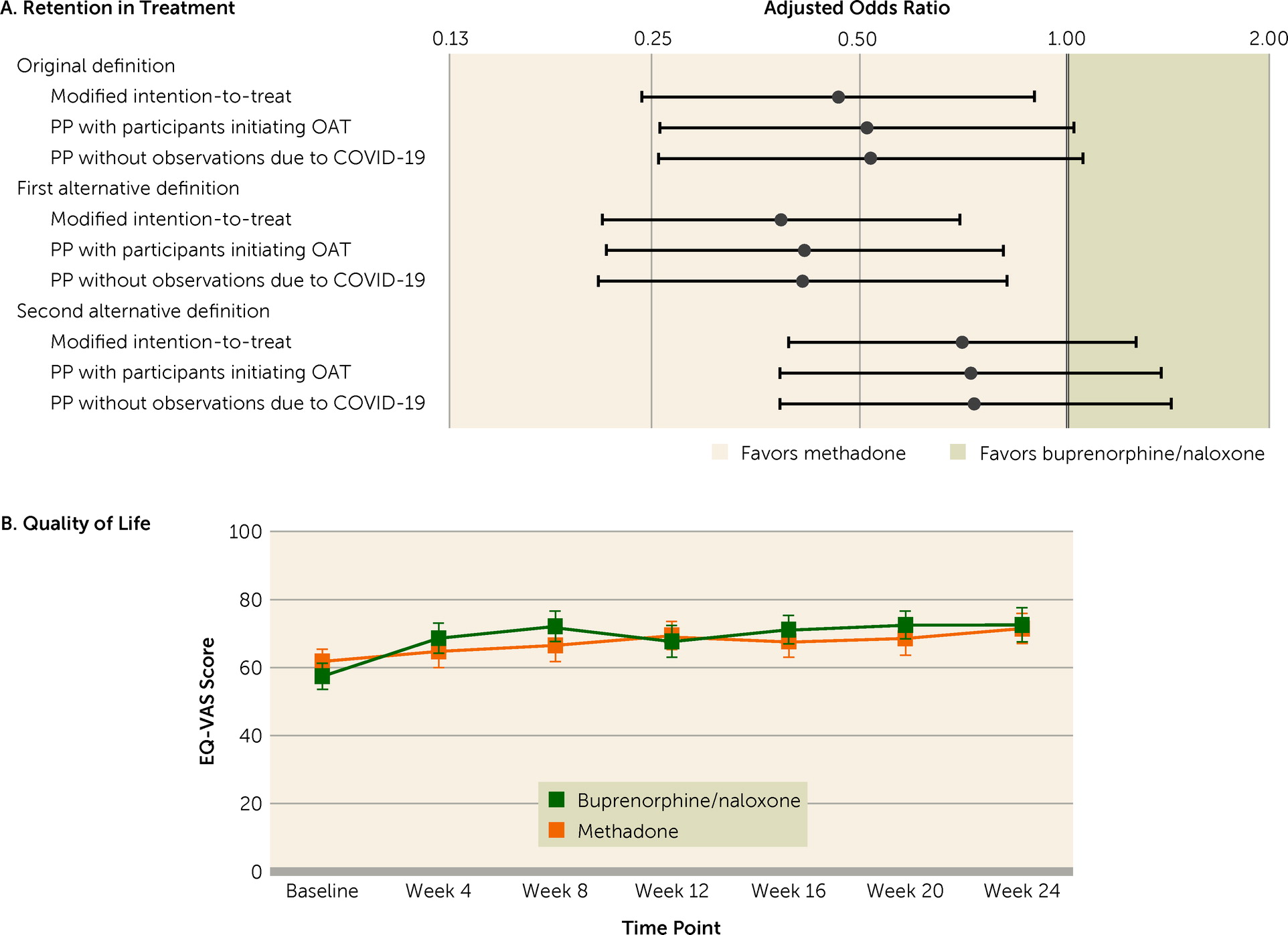
Participants receiving buprenorphine/naloxone had reduced odds of being retained in this treatment compared with those receiving methadone (adjusted odds ratio=0.47, 95% CI=0.24, 0.90, p=0.024), which was also the case when retention was defined as having a prescription at week 24. However, the odds of being retained on any OAT were not statistically different between groups (
Figure 3A,
Table 3).
As illustrated in
Figure 3B, mean quality of life significantly increased in both groups from baseline (buprenorphine/naloxone group, mean=57.0, SD=21.4; methadone group, mean=61.2, SD=20.0) to week 24 (buprenorphine/naloxone group, mean=72.2, SD=20.2; methadone group, mean= 71.0, SD=18.5) (β=9.27, 95% CI=5.14, 13.39, p<0.0001). Although there was no statistically significant group difference (β=−4.20, 95% CI=−8.90, 0.50, p=0.080), there was a statistically significant time-by-treatment interaction (p=0.033). Quality of life differently varied with time depending on treatment, with an initial steeper increase in the buprenorphine/naloxone arm compared with a progressive increase in the methadone arm (
Table 4).
Table 5 lists all drug-related adverse events and their relative risk; Table S2 in the
online supplement lists adverse events and their relative risk during the trial. The most common drug-related adverse events were withdrawal symptoms, overdose, and hypogonadism. The hazard of experiencing any drug-related adverse event or severe adverse event was similar in both groups (hazard ratio=0.84, 95% CI=0.32, 2.25, p=0.73). One death occurred in each group; neither death was associated with the assigned treatment.
Discussion
The results of this pragmatic trial confirmed that a model of care with flexible take-home buprenorphine/naloxone doses was safe and was noninferior to closely supervised methadone treatment in reducing opioid use among Canadians with POUD. This adds to the relatively limited, low-quality evidence available on unsupervised dosing strategies, which were shown not to be significantly different than close supervision for retention and opioid use cessation (
32). In the present study, not only was buprenorphine/naloxone noninferior to methadone with regard to opioid-free urine drug screens, but it was also associated with better outcomes across modified intention-to-treat and sensitivity analyses. Although our results on rates of opioid use were lower than expected, they are in line with those of two meta-analyses (
15,
25) and pragmatic trials supporting noninferiority (
41–
45) or superiority (
46,
47) of buprenorphine over methadone in OUD populations. In addition to buprenorphine/naloxone take-home flexibility, one possible explanation could be related to a quicker reach of therapeutic dosage with buprenorphine/naloxone compared with methadone. Post hoc analysis showed that buprenorphine/naloxone was associated with higher rates of opioid-free urine drug screens at the beginning of the trial, and then was comparable later on. This intrinsic advantage of buprenorphine/naloxone is not trivial, as the early phase of treatment remains a key at-risk period for adverse events, including overdose (
48).
Our results of increased retention in the assigned treatment in the methadone group are also consistent with a meta-analysis of studies conducted in populations with OUD (
25). It is possible that daily treatment supervision increased retention among the generally unstable sample of participants, or that, given the partial agonist nature of buprenorphine, it was perceived as less adequate in the presence of highly potent full-agonist opioids on the market. Alternatively, the similarity of group retention on any OAT and the higher number of treatment switches in the buprenorphine/naloxone group may reflect that buprenorphine/naloxone is easier to transition from than methadone (
25). Buprenorphine’s flexibility in regard to switching to another OAT may be critical for patients who may prefer or clinically require such a transition (
41). This advantage, combined with the tolerability and safety profile of buprenorphine/naloxone, supports its use as a first-line agent.
Beyond such differences, treatment retention and opioid-free urine drug screen rates in both arms were lower than those reported in previous open-label and explanatory trials (
25). This can be explained by the pragmatic design, involving participants with varying living situations, comorbidities, and motivation for treatment. These are typical of real-word clinical populations who are less likely to adhere to treatment compared with the carefully selected samples of efficacy trials. The accessible Canadian health care coverage (which offers many affordable options to access OAT other than through clinical trials, and without randomization), the many COVID-19 pandemic challenges, and the increasingly potent opioids available on the illicit market (
9) may also have lowered retention and opioid-free urine drug screen rates. The low proportion of negative urine drug screens (<10%) among fentanyl users in both treatment arms suggests that the change in opioid potency may contribute to what seems to be a drastic decrease in OAT effectiveness compared with that of trials from recent decades. However, despite frequent ongoing opioid use and some OAT-related adverse events (similar to those previously documented [
30,
44,
49]), participants in both groups improved their quality of life. This suggests that whether or not they were still using opioids, the overall well-being of the study population improved during treatment.
This study has several limitations. First, although an expert consensus was reached for the 15% noninferiority margin, it was subjective in nature. It is unlikely that another choice of margin would have led to a different interpretation of the findings, as our results point toward superiority of buprenorphine/naloxone over methadone. Second, the exclusion of potential participants who had pain that would have required opioids for pain management should also be taken into account when generalizing the results of this trial. Third, the mean therapeutic dosing of methadone was not at the higher end of the possible dosage range for this OAT, which could have decreased its effectiveness. However, although our design restricted us in influencing physicians’ prescribing, our mean dosing of 82 mg was nevertheless higher than that of previous trials (
30,
44,
46,
50–
52). While all modified intention-to-treat and sensitivity analyses were consistent, the high attrition and low retention rates call for cautious interpretation of the results. This is particularly true given our conservative approach of classifying all missed or unavailable urine drug screens as positive in the primary analysis. Our study also included only Canadian sites, with specific facilitators and barriers to care. Therefore, our findings may not apply to other regions, with different conditions.
In conclusion, this study suggests that a buprenorphine/naloxone model of care incorporating a flexible approach to take-home doses is safe and noninferior to methadone for people with POUD, while illustrating that reaching optimal outcomes may be quite difficult in real-world settings. These dynamics are likely to be amplified in the context of the ongoing COVID-19 pandemic. The low rates of retention and success in decreasing opioid use also suggest that the overall efficiency of OAT may be lower than expected, notably in the context of the highly potent opioids frequently available and used by people with POUD. This finding calls for an urgent and much-needed research effort to develop and test innovative strategies to improve treatment outcomes. Integrated psychosocial interventions specifically targeting retention, adjunctive pharmacological treatments, and the development of new, better-adapted models of care may be prime targets for achieving such a goal. Notwithstanding the trial’s inherent limitations and the unique context within which it was conducted, the present study remains timely, as it provides much-needed evidence on the effectiveness of OAT among individuals with POUD, thus informing options for models of care representing real-world clinical settings and practices.
Acknowledgments
The authors are grateful to all study participants and clinical and research staff. The authors especially thank Denise Adams, Oluwadamilola Akinyemi, Farihah Ali, Katrina Blommaert, Emma Garod, Nirupa Goel, Wendy Mauro-Allard, Kirsten Morin, Benita Okocha, Eve Poirier, Aïssata Sako, Geneviève St-Onge, José Trigo, Angela Wallace, and Amel Zertal for research and administrative assistance.