Incorporating Neural Circuit Dysfunction Into the Diagnostic Subtyping of Depressive and Anxiety Disorders: Biotyping Anchored in Neuroimaging
Researchers have identified circuits that are present intrinsically during task-free and at-rest states that are reproducible across studies and thought to underlie fundamental processes of self-reflection, salience perception and attention, and sensation (
Buckner et al. 2013;
Cole et al. 2014). Investigators have also identified circuits evoked by tasks that engage processes of emotional and cognitive function (
Cole and Schneider 2007;
Haber and Knutson 2010;
Niendam et al. 2012). In illustrating a biotype approach to subtyping based on fMRI, we focus on six circuits: default mode, salience, negative affect, positive affect, attention, and cognitive control (
Williams 2016,
2017). Knowledge about how disruptions of these circuits map onto clinical features and treatments is still emerging.
The
default mode circuit (also known as the default mode network) has core connections between the anterior medial prefrontal cortex, posterior cingulate cortex, and angular gyrus (
Greicius et al. 2003,
2009), and is typically assessed in task-free conditions. Disruptions in default connectivity are considered to reflect maladaptive self-referential processes expressed in rumination and worry. Distinct subtypes of depression have been distinguished by both hyperconnectivity (for meta-analysis, see
Kaiser et al. 2016; for review, see
Hamilton et al. 2015) and hypoconnectivity of the default mode (
Price et al. 2017;
Zhu et al. 2012; for meta-analysis, see
Yan et al. 2019).
The
salience circuit has core nodes in the anterior insula, anterior cingulate, and extended amygdala and is thought to detect salient interoceptive and exteroceptive changes. Salience circuit hypoconnectivity has been associated with greater symptom severity (
Goldstein-Piekarski et al. 2020;
Mulders et al. 2015) and may implicate generalized anxiety and anxious avoidance in particular (
Mulders et al. 2015;
Peterson et al. 2014;
Williams 2016). Task-evoked insula hyperreactivity has been observed for sadness and disgust in MDD (
Stuhrmann et al. 2011) and for anger, fear, and happiness in generalized anxiety disorder (
Klumpp et al. 2013), suggesting in part a bias toward mood-congruent negative stimuli.
Affective circuits are robustly activated by stimuli that signal potential threats, negative events, or rewards. The
negative affect circuit comprises the amygdala and connections with medial cortical regions, including ventral and dorsal medial prefrontal and anterior cingulate regions. Amygdala hyperreactivity occurs in depressive disorder, generalized anxiety disorder, social phobia/anxiety, and panic disorder elicited by threat-related stimuli (
Fonzo et al. 2015;
Jaworska et al. 2015;
Killgore et al. 2014), and in depressive disorder elicited by sad stimuli (
Williams 2016). Alterations in activation may also reflect a reduction in connectivity between the amygdala and regions of the anterior cingulate and medial prefrontal cortex (
Matthews et al. 2008;
Prater et al. 2013).
Two additional circuits are relevant to the cognitive and concentration features of depression and anxiety, which are commonly given less emphasis than mood features. The frontoparietal
attention circuit has been identified in the task-free state and is defined by core regions in the superior frontal cortex and anterior inferior parietal lobe, connecting with frontal eye fields. Relative hypoconnectivity within this circuit, and within constituent regions, has been implicated in the inattention and accompanying cognitive symptoms common across mood and anxiety disorders (
Goldstein-Piekarski et al. 2020;
Keller et al. 2020). The executive, or
cognitive control, circuit involves the dorsal components of the lateral prefrontal cortex (dorsolateral prefrontal cortex [DLPFC]), anterior cingulate cortex (dACC), and parietal cortex engaged by tasks that require higher cognitive functions such as working memory and selective control of cognition (
Niendam et al. 2012). In depression and social anxiety, DLPFC and dACC hypoactivation has been observed during cognitive tasks and in stress-induced situations (
Korgaonkar et al. 2013; for review, see
Williams 2016).
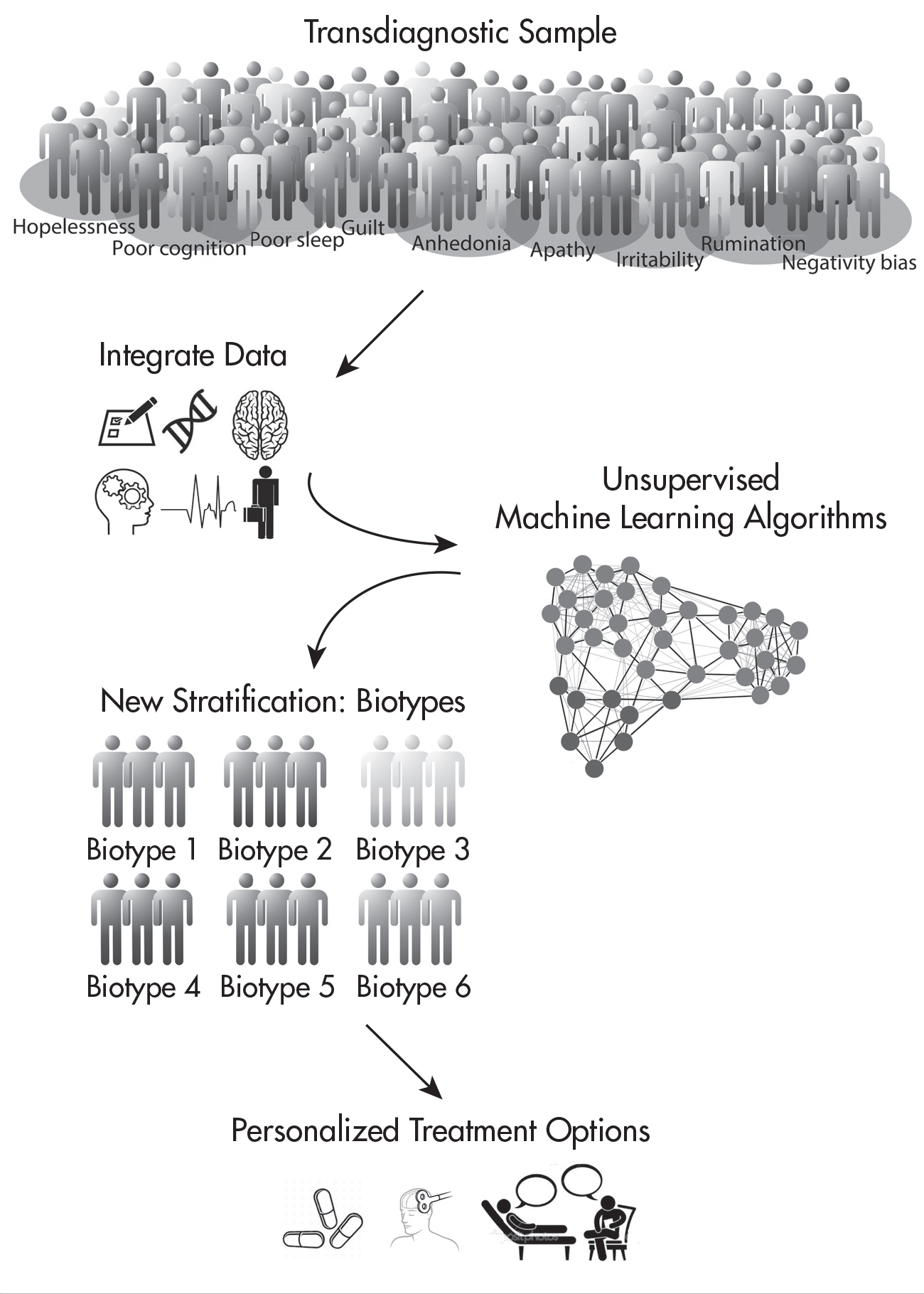
We anticipate that these circuit dysfunctions are modulated and refined as a result of other biological and environmental factors such as genetic variants and exposure to stress. As data accumulate, data-driven approaches will help define the optimal number of biotypes that account for the heterogeneity of mood and anxiety disorders (
Figure 1–1). The utility of such pproaches has been demonstrated for subtypes of depression defined specifically by task-free circuit connectivity (
Clementz et al. 2016;
Drysdale et al. 2017;
Goldstein-Piekarski et al. 2020;
Maron-Katz et al. 2020).
Incorporating Neural Circuit Dysfunction Into Treatment Planning for Depressive and Anxiety Disorders
A goal of using neuroimaging to achieve more precise diagnosis of mood and anxiety disorder subtypes, based on underlying neural circuit function, is to provide clinicians with additional data to inform treatment choices (e.g., identifying which patients may benefit from pharmacotherapy, selecting a pharmacotherapy, limiting side effects). This goal has motivated large biomarker discovery trials that deploy neuroimaging along with other biomarker measures (
Dunlop et al. 2012;
Grieve et al. 2013;
Trivedi et al. 2016).
In the International Study to Predict Optimized Treatment for Depression (iSPOT-D), for example, remission on typical first-line antidepressants depended on intact pretreatment connectivity of the default mode, whereas nonremission was predicted by hypoconnectivity (
Goldstein-Piekarski et al. 2018;
Korgaonkar et al. 2019). For the negative affect circuit evoked by emotion stimuli, pretreatment amygdala hyporeactivity to threat was a general predictor of subsequent response to a selective serotonin reuptake inhibitor (SSRI), whereas hyperreactivity to sadness was a specific predictor of nonresponse to the serotonin-norepinephrine reuptake inhibitor venlafaxine-XR (
Williams et al. 2015). For the cognitive control circuit, intact activation and connectivity were predictive of response to SSRIs (
Gyurak et al. 2016;
Tozzi et al. 2020), while functional connectivity evoked by cognitive inhibition of task responses was shown to specifically differentiate responders to sertraline versus venlafaxine (
Tozzi et al. 2020). In line with our earlier point about the modulation of these circuit dysfunctions, early life stress not only determines poor antidepressant responses overall (
Williams et al. 2016a) but also, when combined with pretreatment negative affect circuit dysfunction, boosts the accuracy for identifying nonresponders (
Goldstein-Piekarski et al. 2016).
Imaging biomarker studies that have formally evaluated sensitivity and specificity for first-line antidepressants have observed a predictive accuracy for response or remission of 70% or greater, which suggests these biomarkers have clinical utility. Although these figures may be reduced following external validation studies (i.e., when replications are attempted in independent samples), they still reflect great promise. Given that current treatment-matching approaches are essentially trial and error and a majority of patients do not respond to their first medication, even a small increase in predictive accuracy would be worthwhile. Furthermore, there is minimal risk in a novel strategy for selecting between FDA-approved treatments of comparable overall efficacy. Ultimately, the most valuable clinical predictions will be those that enable patients and providers to differentially select between treatment options.
The findings presented above also highlight the importance of identifying biomarkers that help guide the choice of alternative treatments for patients likely to be non-responders or who are already treatment resistant. Because of an arguably direct impact on large-scale neural circuits, transcranial magnetic stimulation (TMS), an FDA-cleared intervention for treatment-resistant depression, is of great interest in identifying circuit-based biotypes that discern patients who may not respond to pharmacotherapy but may benefit from TMS. Notably, default mode hypoconnectivity (predictive of pharmacotherapy nonremission in iSPOT-D) has been found to characterize responders to TMS (
Philip et al. 2018). Hypoconnectivity of other circuits, such as the positive affect circuit, observed in the task-free state and implicated in pharmacotherapy nonresponse, also shows promise in identifying responders to TMS (
Avissar et al. 2017;
Downar et al. 2014). A disruption in the optimal anticorrelation between connectivity of the default mode (particularly the anterior portion) and the cognitive control circuit may characterize responders to TMS (
Fox et al. 2012;
Weigand et al. 2018). An accelerated form of TMS that targets this disruption may help regulate a more optimal anticorrelation between default mode and DLPFC connectivity and improve remission rates (
Cole et al. 2020). See
Chapter 2 (“The Future of Precision Transcranial Magnetic Stimulation in Psychiatry”) for a more detailed discussion of baseline neuroimaging predictors of response and changes in functional connectivity as a result of TMS therapy.
Another promising avenue for neuroimaging biotypes and biomarkers is the rapid testing of new, alternative pharmacotherapies that have more targeted mechanisms of action. Reward circuit dysfunction and anhedonia do not appear to be modulated by typical antidepressants but offer candidates for novel therapies. For example, preclinical studies have found that the kappa opioid receptor (KOR) antagonist JNJ-67953964 is a promising candidate to modulate reward circuit dysfunction and anhedonia (
Krystal et al. 2018). In a landmark study,
Krystal and colleagues (2020) showed that targeting KOR antagonism with this drug in a transdiagnostic sample of patients with high anhedonia increased ventral striatal activation and concurrently improved anhedonia symptoms. A natural next step would be to assess whether KOR antagonism therapy preferentially improves outcomes when deployed in a stratified design in which individuals are preselected according to ventral striatal function (
Williams and Hack 2020).
Accelerating the Clinical Translation of Neuroscience-Informed Precision Psychiatry
Precision psychiatry is not yet a clinical reality. Here, we outline three related initiatives we have launched at Stanford to accelerate progress in the clinical translation of precision psychiatry informed by neuroscience.
DISCOVERY CLINIC FOR NEUROSCIENCE-INFORMED PRECISION PSYCHIATRY
In 2013, LMW launched partnerships between her research lab at Stanford and two local area clinics: a community mental health center encompassing clinics for mood and anxiety issues with a combined focus on clinical training, and a technology-enabled health care company integrating mental health with primary care. These partnerships were centered around a project funded by the National Institute of Mental Health under the Research Domain Criteria (RDoC) initiative. This project recruited participants in 2013–2016 who were experiencing a range of palpable symptoms related to states of negative affect, unmedicated at the time of the study, and who completed functional neuroimaging as well as symptom, cognitive, daily function, and coping assessments (
Williams et al. 2016b).
To initiate an understanding of the clinical utility of the RDoC approach, which is anchored in the neuroscience dimensions that underlie psychiatric disorders, LMW embedded a Discovery Clinic within the project flow. The Discovery Clinic consisted of several voluntary and confidential components included in the institutional review board–approved overall protocol: a feedback session after baseline assessments, a 12-week follow-up, quarterly meetings to discuss and refine the processes, and didactic sessions for clinic trainees. In feedback sessions involving LMW, the usual-care clinician, and the participating patient, LMW discussed a “beta” report that provided information about each patient’s profile of symptoms, as well as cognitive and daily function data and information about fMRI. Clinician and patient could ask questions regarding the possible meaning of the information given the current state of scientific knowledge. Clinicians chose the extent to which they incorporated information into their ongoing case formulation process, then discussed the combined information and any implications for treatment choice to commence at their subsequent ongoing clinical sessions (at which LMW was not present). As the individualized fMRI data were refined as part of the ongoing parallel research project, they were also made available.
At the 12-week follow-up, symptom and daily function assessments were repeated, providing a naturalistic means to evaluate clinical outcomes. A debrief about the experience was also completed at this time via a brief survey and a video link with clinicians who provided qualitative feedback on each patient’s experience. Fifty-one feedback sessions were completed, which identified two common themes: 1) the value to the clinician of having access to multiple sources of information perhaps not apparent in the clinical interview process (e.g., evidence of cognitive impairment, or evidence of extreme anhedonia even in the absence of overall severity of symptoms and knowledge about neural circuit dysfunction), and 2) the destigmatizing and demystifying experience of “seeing” individualized report information as described by participants. Indeed, having a shared tangible model of understanding for patients potentially provides a narrative that diminishes shame and self-blame, especially when the underlying biology is modifiable by interventions.
We present below two case illustrations of participants who received feedback from LMW and whose treatment plans were modified by the clinician after their sessions.
Clinical Case Illustrations
Mr. RT, a male engineer in his 50s with recurrent MDD, was unable to continue working due to the stress of his recent promotion. Six treatment trials (five SSRIs and electroconvulsive therapy) had failed. Mr. RT exhibited prominent anhedonia and hopelessness, while his cognitive testing revealed a slowed reaction to identifying happy faces. Increased time to identify happy emotions has been associated with symptoms of anhedonia (
Vrijen et al. 2016). Mr. RT’s biotype profile showed the greatest dysfunction in reward neurocircuitry. The ventral striatum, a key node in the reward circuitry, was shown to be hypoactive in neuroimaging studies of depressed patients who endorsed prominent anhedonia (
Der-Avakian and Markou 2012;
Greenberg et al. 2015). In developing a treatment plan in light of information from the feedback report, Mr. RT’s mental health team considered prior evidence from a target engagement study that the selective D
3 dopamine receptor agonist pramipexole increased activation in a key region of the ventral striatum (
Ye et al. 2011). Mr. RT tried pramipexole, and his anhedonia improved within 4 weeks. This improvement was maintained throughout a 16-week follow-up period.
Ms. B, a female college freshman, had a diagnosis of MDD at the time of her feedback session with LMW. Multiple SSRIs had failed to elicit a response, and she was not interested in trying another medication. Ms. B had a history of psychiatric hospitalization for active suicidality, and her symptom questionnaires indicated that she had prominent worry, rumination, self-blame, and poor sleep. Her imaging data revealed default mode disruptions implicating poor response to antidepressants (
Goldstein-Piekarski et al. 2018). Given emerging evidence that default mode disruptions implicated in poor response to antidepressants may be associated with good response to TMS (
Philip et al. 2018), and clinical information available to the patient’s treatment team, this treatment team opted for an accelerated form of TMS. This treatment led to remission of MDD within 1 week, with the MDD still in remission during study follow-up and at a subsequent 10-month check-in.
INTEGRATING THE DISCOVERY CLINIC WITH RESIDENT TRAINING PROGRAMS
In 2017, building from the overall positive response of the initial Discovery Clinic process, LMW launched what is, to our knowledge, the first “Discovery Training Clinic” of its kind, a collaboration between researchers, educators, and clinicians in the Stanford Department of Psychiatry and Behavioral Sciences. The goal was to further inform the clinical translation of neuroscience-informed precision psychiatry by incorporating the principles of this approach directly into the clinical training of psychiatry residents. With the support of the Chief of Adult Psychiatry and Residency Training leadership (Director, Chris Haywood, M.D., and Assistant Director, Belinda Bandstra, M.D. [BB]), LMW designed and piloted a pragmatic clinical translational program that incorporated the feedback session principles from the initial Discovery Clinic process and the content of prior feedback sessions to develop structured case examples for teaching. TMB joined LMW to implement the program within the context of a yearlong third-year resident (PGY3) training rotation within the departmental Continuity Clinic. Over the year, researchers (TMB, LMW), residents, BB, and attendings met with two subgroups of approximately three residents each on alternating weeks. The program comprised three primary components: structured discussion of case examples based on prior cases, open discussion of case formulation issues (such as how to incorporate neuroscience measures into the clinical decision-making process), and discussion of new feedback session data from the residents’ own consenting patients.
Participating patients (28 referred, 20 enrolled) undertook the same neuroimaging, cognitive, symptom, and function assessments as per the initial RDoC project–related Discovery Clinic. To facilitate learning about the impact of both residents and patients receiving neuroscience-related information, we randomized the feedback process so that half of the time residents (with attending) received the report prior to their first clinical appointment with the patient, and the other half of the time they received the report 12 weeks later. We evaluated the program after the first year. Residents indicated the experience was useful but expressed the need for the neuroscience information to be sequenced ahead of the direct clinical application, especially because they were new to the independent implementation of clinical decision making. Most patients agreed that the report information helped them understand how their brain functioned, provided new insights into their symptoms, and enabled them to feel more committed to treatment. Thus, in 2019, LMH joined the Discovery Clinic and with TMB further refined and expanded the program to include additional structured case studies and a didactic curriculum.
THE STANFORD TRANSLATIONAL PRECISION MENTAL HEALTH CLINIC
In 2018, LMW founded the Stanford Center for Precision Mental Health and Wellness, which has the translational goal of accelerating the insights of the Discovery Clinic into practice.
Then, in 2021, LMH and LMW launched the Stanford Translational Precision Mental Health Clinic; LMH is the director, and LMW serves as an expert advisor to the clinic regarding the imaging and biotype information. The goal of this translational consultation clinic is to offer a cutting-edge, multimodal assessment for treatment-resistant patients with mood and/or anxiety disorders in order to help better match their biological subtype to treatment. Any patient qualifying for one of our research studies has the opportunity to learn more about the clinic. Similar to our Discovery Clinic, participating patients undergo a comprehensive battery of evaluations assessing symptoms, neurocognition, pharmacogenetic variants, resting and task-based fMRI, and blood-based markers. All patients receive a report of the findings, along with a thorough explanation and their implications for treatment recommendations. This information is also discussed with their referring provider. Our hope is that, through this experimental approach, we may help relieve some of the tremendous suffering that is a consequence of our current trial-and-error approach to mental health treatment.
Conclusion and Future Directions
We envision a future that overcomes the gaps between research advances and their application in practice. New knowledge about neural circuits will be incorporated into models of assessment and care delivery, residency programs will prepare graduates with training in neuroscience, and clinicians will have access to neuroscience-based tools to inform their decision making as part of the routine, reimbursable workflow. We can foresee having a clinical toolkit that is the psychiatry equivalent of cardiology: multiple imaging modalities that help differentially diagnose the source of the underlying pathophysiology and guide choice of treatments accordingly, including lifestyle changes, medications, behavioral therapies, neuromodulation, and their combination. With such a precision approach that translates brain insights into clinically actionable tools, we have the opportunity to improve and save the lives of many.