Augmentation Strategies for Treatment-Resistant Anxiety Disorders: A Systematic Review and Meta-Analysis
Abstract
Background:
Methods:
Results:
Conclusions:
Background
Methods
Search Methods to Identify Studies
Data Collection and Analysis
Results
Results of the Search
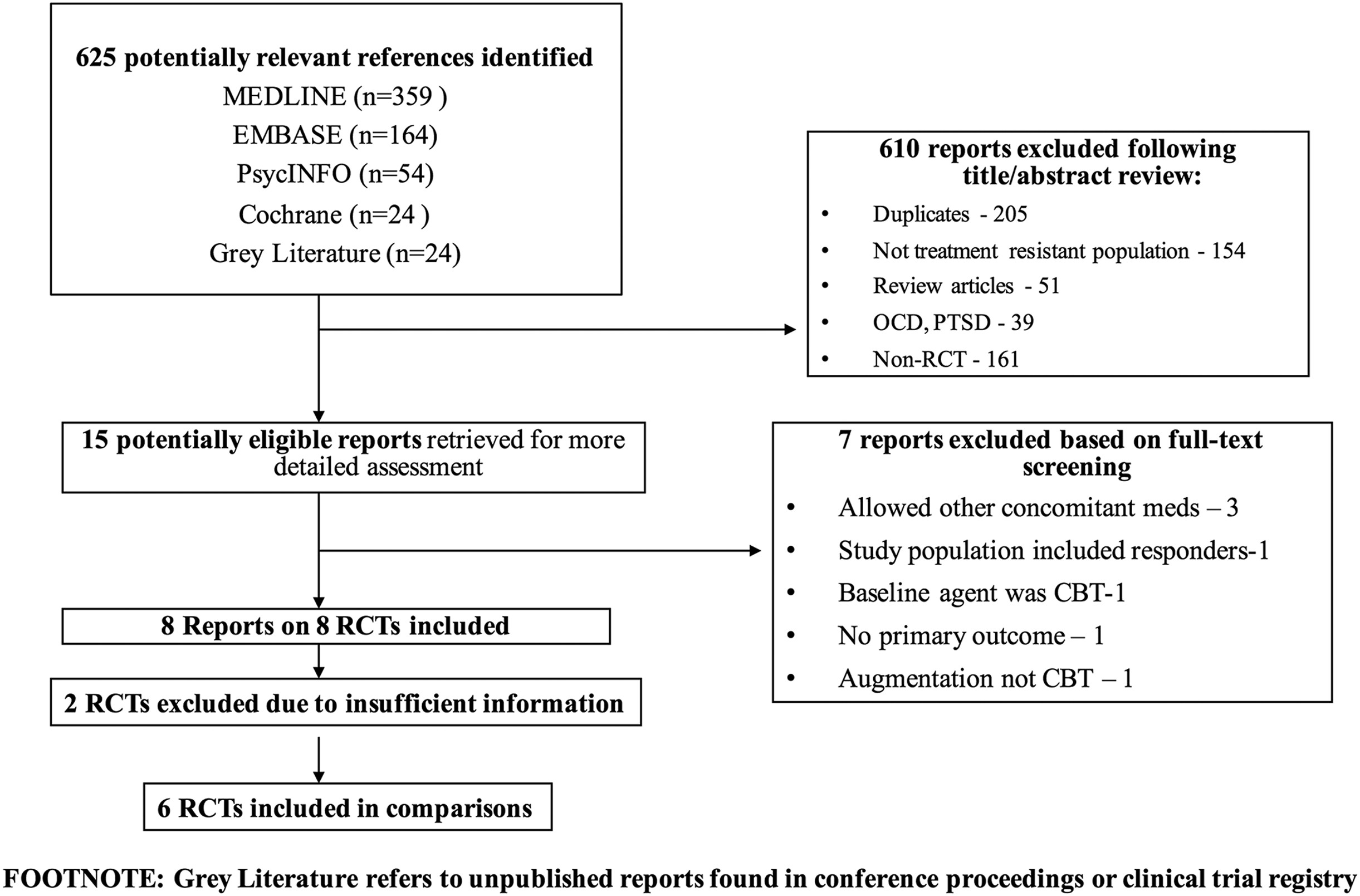
Included Studies
| Included studies | |||||||
|---|---|---|---|---|---|---|---|
| Study | Disorder | Design | Treatment (N) | Control (N) | Study duration (weeks) | Mean age ± SD | Publication type |
| • Augmentation Agent | |||||||
| • Type of control | |||||||
| • Parallel/cross-over | |||||||
| Pollack et al.[17] | GAD | Olanzapine | 9 | 12 | 6 | 43.8 ± 14.90 | Published report |
| Placebo Parallel | |||||||
| Altamura et al.[18] | GAD | Quetiapine | 10 | 10 | 8 | 49.20 ± 14.72 | Published report |
| Placebo Parallel | |||||||
| Rickels et al.[19] | GAD | Pregabalin | 177 | 176 | 8 | 43.7 ± 11.5 | Published report |
| Placebo Parallel | |||||||
| Pollack et al.[20] | SAD | Clonazepam | 63 | 59 | 12 | 35.50 ± 13.0 | Published report |
| Placebo Parallel | |||||||
| Simon et al.[21] | Panic disorder | Clonazepam | 9 | 10 | 12 | 37.7 ± 11.2 | Published report |
| CBT Parallel | |||||||
| Goddard et al.[22] | Panic disorder | Quetiapine | 13 | 13 | 8 | 36.0 ± 13.0 | Poster-ADAA 2012 |
| Placebo Parallel |
| Excluded studies | |||||||
|---|---|---|---|---|---|---|---|
| Study | Disorder | Design | N | Study duration (weeks) | Reason for exclusion | ||
| • Augmentation Agent | |||||||
| • Type of control | |||||||
| • Parallel/cross-over | |||||||
| Brawman-Mintzer et al.[25] | GAD | Risperidone (atypical antipsychotic) | 40 | 6 | • Wide range of baseline and concomitant agents | ||
| Placebo Parallel | |||||||
| Pandina et al.[26] | GAD | Risperidone (atypical antipsychotic) | 417 | 4 | • Wide range of baseline and concomitant agents | ||
| Placebo Parallel | |||||||
| Simon et al.[27] | GAD | Quetiapine (atypical antipsychotic) | 22 | 18 | • Study population—remitters (responders included) | ||
| Placebo Parallel | |||||||
| Lohoff et al.[28] | GAD | Ziprasidone (atypical antipsychotic) | 62 | 8 | • Wide range of baseline and concomitant agents | ||
| Placebo Parallel | • Only some pts were augmented | ||||||
| Schutters et al.[29] | SAD | Paroxetine (SSRI) | 21 | 24 | • Augmentation phase of study not RCT | ||
| Placebo Parallel | |||||||
| Hirschman et al.[30] | Panic disorder | Pindolol (beta-blocker) | 25 | 12 | • No primary outcome measure identified | ||
| Placebo Parallel | • No definition of response | ||||||
| • Did not characterize dropouts (i.e., whether from treatment or placebo arms) | |||||||
| Kampman et al.[31] | Panic disorder | Paroxetine (SSRI) | 161 | 8 | • Baseline agent was CBT | ||
| Placebo Parallel | |||||||
Trials Excluded from the Review
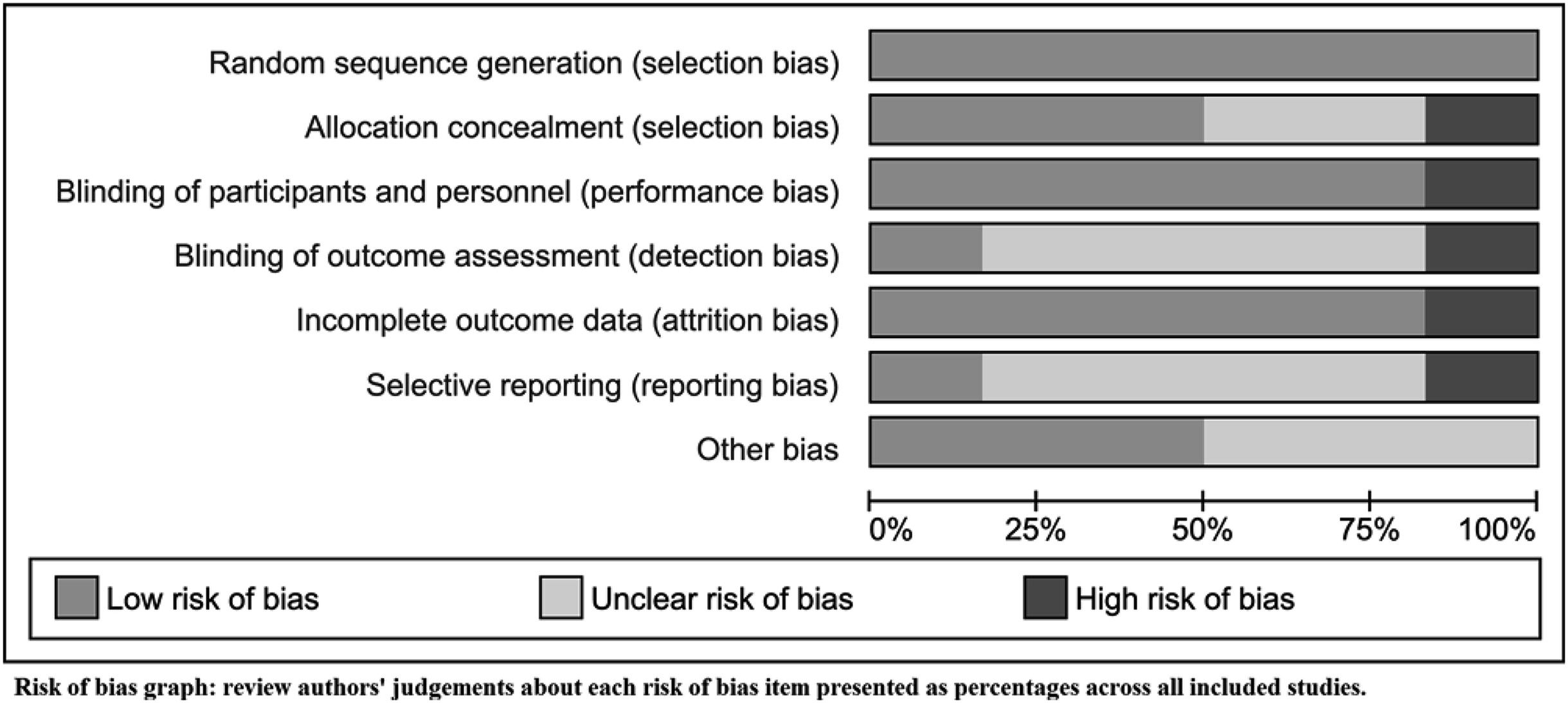
Risk of Bias in Included Studies
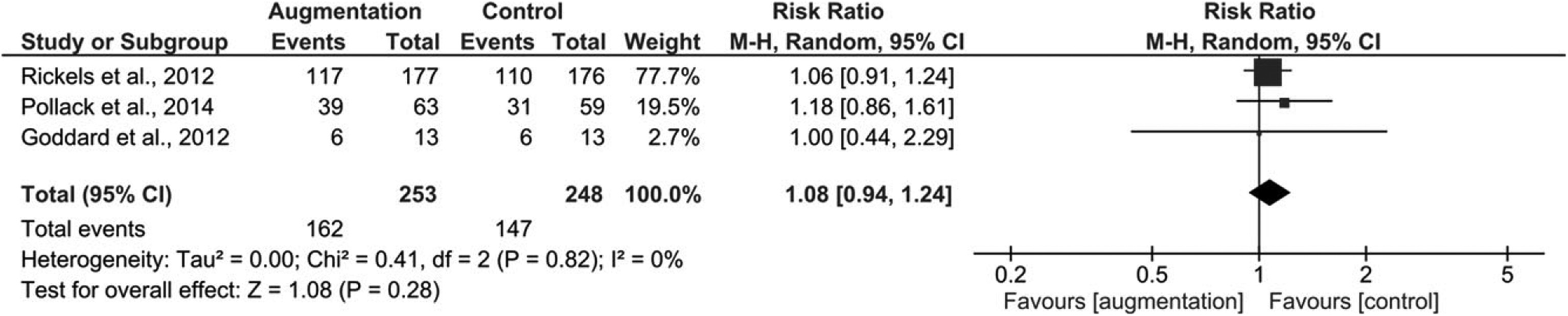
Primary Outcome
Treatment Response.
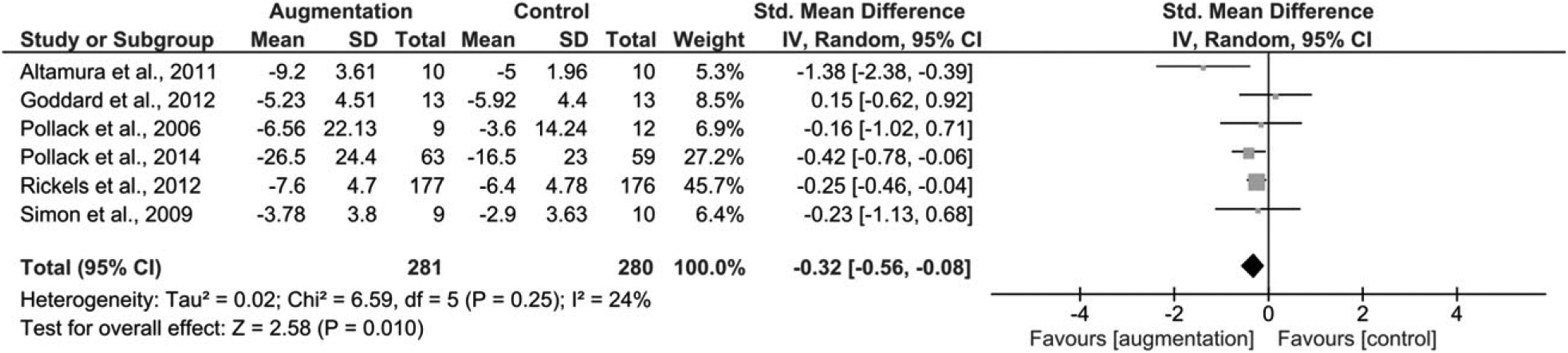
Secondary Outcomes
Reduction in Symptom Severity.
Disability/Functional Impairment
Dropouts Due to Adverse Events
Discussion
Strengths and Limitations
Research and Clinical Implications
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBGet Access
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

