Updates in the Assessment and Management of Agitation
Abstract
Assessment
Risk Factors and Acute Presentations
BOX 1. Modifiable and nonmodifiable risk factors for agitation
Nonmodifiable Risk Factors
Modifiable Factors
Acute Clinical Presentations of Agitation
Screening and Rating Scales for Agitation
| Scale and setting | Type of screening | Comments |
|---|---|---|
| Psychiatric emergency service and emergency department (ED) | Quick screening | High-acuity setting |
| Agitated Behavior Scale (15, 16) | 14 items rated on a 4-point scale of present to absent | Clinician rated, developed for traumatic brain injury, can be used in the ED |
| Behavioural Activity Rating Scale (4, 11) | Single descriptor, seven levels (agitated to sedated) | Observational, easy to use in nonpsychiatric settings or ED |
| Sedation Assessment Tool (17) | 7-point scale of agitation to sedation in two descriptors (speech and responsiveness) | Quick, adaptation of altered mental status score, can be used in the ED |
| Inpatient setting | Longitudinal screening | Inpatient and long-term care |
| Aggressive Behavior Scale (15) | Four-item summary scale | Scored over 7 days, best for inpatient and long-term care |
| Cohen-Mansfield Agitation Inventory (9) | 29 behaviors rated on a 7-point severity scale | Labor intensive, scored over 2 weeks, caregiver rated |
| Richmond Agitation Sedation Scale (4, 18) | 10-point scale (agitation to sedation) in two descriptors | Quick delirium screen for critical care setting, repeat over time |
Etiologies Influencing Agitation
Physical illness and delirium.
| Type | Examples |
|---|---|
| Neurological | Traumatic brain injury, intracranial hemorrhage, seizure, cardiovascular accident, transient ischemic attack, encephalopathies (such as hepatic or renal), autoimmune encephalitis |
| Infectious | Encephalitis, meningitis, sepsis, urinary tract infection or urosepsis (elderly), pneumonia |
| Gastrointestinal or metabolic | Electrolyte disturbances, hypoglycemia, diabetic ketoacidosis, ileus, inflammatory bowel disease, chronic malnutrition, gastrointestinal obstruction |
| Respiratory | Hypoxia, pulmonary embolism, respiratory failure |
| Cardiac | Arrhythmia, myocardial infarction, heart failure |
| Toxicology | Environmental toxins, medication reactions, neuroleptic malignant syndrome, serotonin syndrome |
| Endocrine | Thyroid (thyrotoxicosis, myxedema) |
| Hematologic or oncologic | Anemia, oncologic process |
| Constitutional | Poor sleep, pain, hypothermia, or hyperthermia |
Substance use and withdrawal.
Psychiatric and developmental conditions.
Management
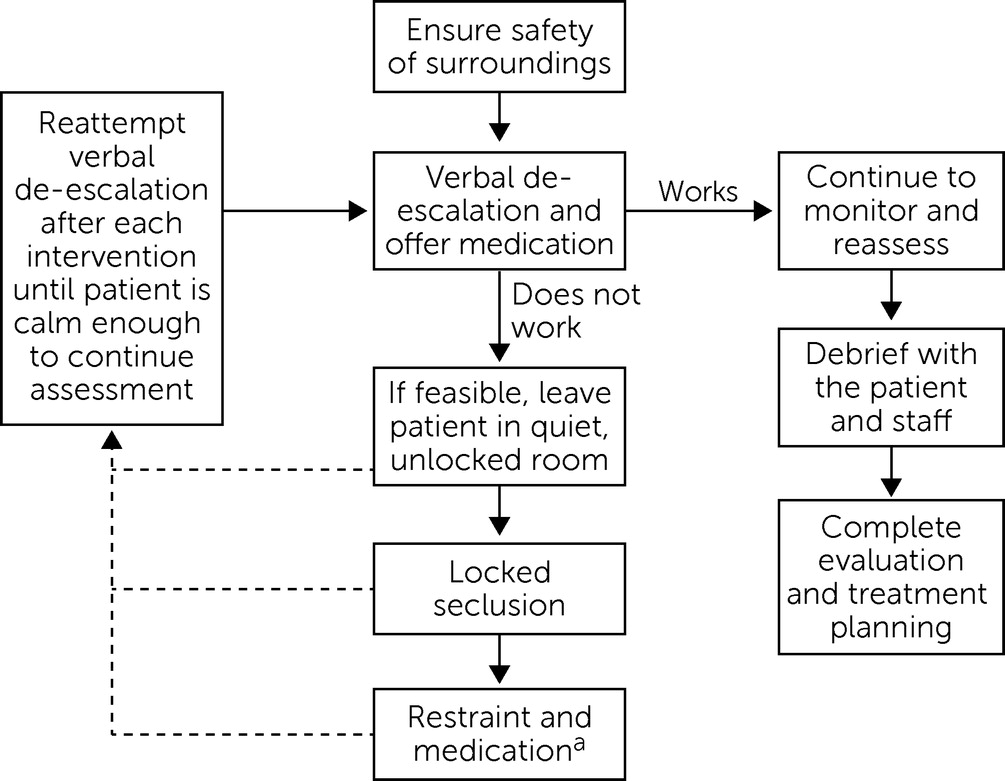
Nonpharmacological Interventions
| Skill | Comments |
|---|---|
| Respect personal space | Maintain at least two arms’ lengths (6 feet) and allow space for both you and patient to exit |
| One person | Designate one person to engage the patient; introduce yourself and your role |
| One message | Be concise, speak clearly and simply, repeat instructions multiple times |
| Name feelings | Listen closely to try to identify and validate the patient’s emotional experience; share how the patient’s behavior is affecting you; reflect what you are hearing to clarify emotions and meaning behind statements shared |
| Appeal to the patient’s wants or needs | Listen for anything you may be able to offer the patient, such as a psychotherapeutic intervention or a comfort item; focus on what you can do for the patient |
| Set limits and reinforce appropriate behavior | Respectfully communicate your expectations and the consequences of unacceptable behaviors; focus on shared values, such as safety, respect, and improvement in the patient’s quality of life or functioning |
| Offer choices | Ask the patient to consider what has worked in the past; offer choices between medications or route of administration |
Pharmacological Management
| Suspected etiology | Medication and dose | Comments |
|---|---|---|
| Primary psychiatric or undifferentiated with prominent psychosis | Olanzapine, 5 mg–10 mg (PO, ODT or IM); risperidone, 2 mg (PO, ODT or liquid); ziprasidone, 10 mg–20 mg (IM); haloperidol, 2 mg–10 mg (PO or IM); droperidol, 5 mg–10 mg (IM) | Add lorazepam 1 mg–2 mg if haloperidol or monotherapy ineffective; risk of respiratory depression for IM olanzapine and IM lorazepam within 1 hour |
| Intoxication with central nervous system depressant (including alcohol) | Haloperidol, 2 mg–10 mg (PO or IM); olanzapine, 5 mg (PO); risperidone, 2 mg (PO, ODT or liquid) | Avoid benzodiazepines if possible; FGAs still heavily favored, but SGAs likely safe if given PO; IM risks respiratory depression |
| Stimulant intoxication, alcohol or benzodiazepine withdrawal OR undifferentiated without psychosis | Lorazepam, 1 mg–2 mg (PO or IM); diazepam, 5 mg–10 mg (PO); midazolam, 5 mg–10 mg (IM) | Add SGA if prominent psychotic symptoms |
| Delirium | Risperidone, 1 mg–2 mg (PO, ODT); olanzapine, 5 mg–10 mg (PO, ODT or IM); haloperidol, <5 mg (PO or IM); ziprasidone, 10 mg–20 mg (IM) | Avoid benzodiazepines; high risk of EPS with haloperidol doses >3 mg; consider QTc monitoring |
Antipsychotics.
Benzodiazepines.
Other medications or formulations.
Restraint and Seclusion
Evaluation and Management of Agitation in Youths
Evaluation
| Screening tool | Description | Comments |
|---|---|---|
| Abbreviated Brief Rating of Aggression by Children and Adolescents (55) | 14-item instrument | Used to predict potential for agitation and aggression on an inpatient unit |
| Dynamic Appraisal of Situational Aggression (inpatient) (56, 57) | Seven items rated present or absent | Assesses likelihood of aggression in the next 24 hours |
| Modified Overt Aggression Scale (58, 59) | Four-part behavioral rating scale; four types of agitation or aggression rated over past week | Inpatient or residential setting |
Nonpharmacological Interventions
Pharmacological Management
| Medication | Dose | Peak effect (minutes) | Maximum daily dose (mg by weight or age) | Other considerations |
|---|---|---|---|---|
| Clonidine | PO, 0.05 mg–0.1 mg | 30–60 | <40 kg, 0.2 mg; >40 kg, 0.3 mg– 0.4 mg | Monitor for hypotension and bradycardia |
| Diphenhydramine | PO or IM, 12.5 mg– 50 mg or 1 mg per kg body weight | 120 | Age <12, 50 mg– 100 mg; age >12, 100 mg–200 mg | Avoid in delirium and in youths at risk of paradoxical reaction (e.g., autism); can use to mitigate EPS risk from antipsychotics |
| Lorazepam | PO, IM, or IV, 0.5 mg– 2 mg or 0.05 mg– 0.1 mg per kg body weight | PO, 60–120; IM or IV, 10 | Age <12, 4 mg; age >12, 6 mg– 8 mg | Avoid in delirium and in youths at risk of paradoxical reaction (e.g., autism); can use to mitigate EPS risk from antipsychotics |
| Chlorpromazine | PO or IM, 12.5 mg– 60 mg or 0.55 mg per kg body weight | PO, 30; IM, 15 | Age <5, 40 mg; age >5, 75 mg | Monitor for hypotension; IM dose half of PO dose |
| Haloperidol | PO or IM, 0.5 mg– 5 mg or 0.55 mg per kg body weight | PO, 30; IM, 15 | <40 kg, 6 mg; >40 kg, 15 mg | Monitor for hypotension; monitor EKG, particularly with IM or IV use; IM dose half of PO dose; EPS risk lower with IV use |
| Olanzapine | PO or IM, 2.5 mg– 10 mg | PO, 240–480; IM, 15–45 | Age > 12, 20 mg | ODT formulation available; do not give IM within 1 hour of IM or IV benzodiazepines |
| Risperidone | PO, 0.25 mg–1 mg or 0.005 mg–0.01 mg per kg body weight | 30–60 | Age <12, 1 mg–2 mg; age >12, 2 mg–4 mg | ODT formulation available; watch for EPS |
| Quetiapine | PO, 25 mg–50 mg or 1 mg–1.5 mg per kg body weight | 30 | Age >12, 600 mg– 800 mg | Monitor for sedation and hypotension |
| Etiology | Evaluation considerations | Pharmacological management |
|---|---|---|
| Delirium | Regular medical evaluation; environmental modification | Avoid benzodiazepines and anticholinergics; PO, clonidine, quetiapine, risperidone, and olanzapine; IM or IV, olanzapine and haloperidol |
| Substance intoxication or withdrawal | History, physical examination, urine toxicology to guide understanding of potential substances implicated | Alcohol or benzodiazepine withdrawal, lorazepam with or without haloperidol; alcohol or benzodiazepine intoxication, haloperidol or chlorpromazine; opiate withdrawal, clonidine, opiate replacement, or supportive treatment; stimulant or phencyclidine intoxication, lorazepam with or without haloperidol |
| Developmental delay or autism | Behavioral intervention; assess for sources of pain or discomfort; support communication; address sensory factors | Benzodiazepines, antihistamines may cause paradoxical response; PO, alpha-2 agonist or antipsychotic; IM, olanzapine or chlorpromazine |
| Primary psychiatric diagnosis | Clarify diagnostic formulation | Catatonia, lorazepam; anxiety or trauma, lorazepam or clonidine; ADHD, alpha-2 agonist, antihistamine, or antipsychotic; oppositional defiant or conduct disorder, antipsychotic or lorazepam; mania or psychosis, antipsychotic with lorazepam or diphenhydramine if concern for EPS |
| Unknown or mixed etiology | Reinforce environmental and behavioral strategies while searching for etiologic factors | Mild or moderate, diphenhydramine, lorazepam, or olanzapine; moderate or severe, chlorpromazine, haloperidol, olanzapine, or lorazepam |
Future Directions
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

