Posttraumatic stress disorder (PTSD) is defined clinically by elevated and sustained symptoms of reexperiencing, hyperarousal, avoidance, mood alterations, and dissociation following the experience of one or more traumatic events (
1). The symptom presentations of PTSD are heterogeneous and have expanded considerably from
DSM-IV to
DSM-5 (
2,
3). The individual differences, spanning genetic, environmental, physiological, and neurobiological factors that contribute to the unique symptom presentations, are not yet well understood but likely entail large-scale interactions across multiple body systems involved in threat processing (
4).
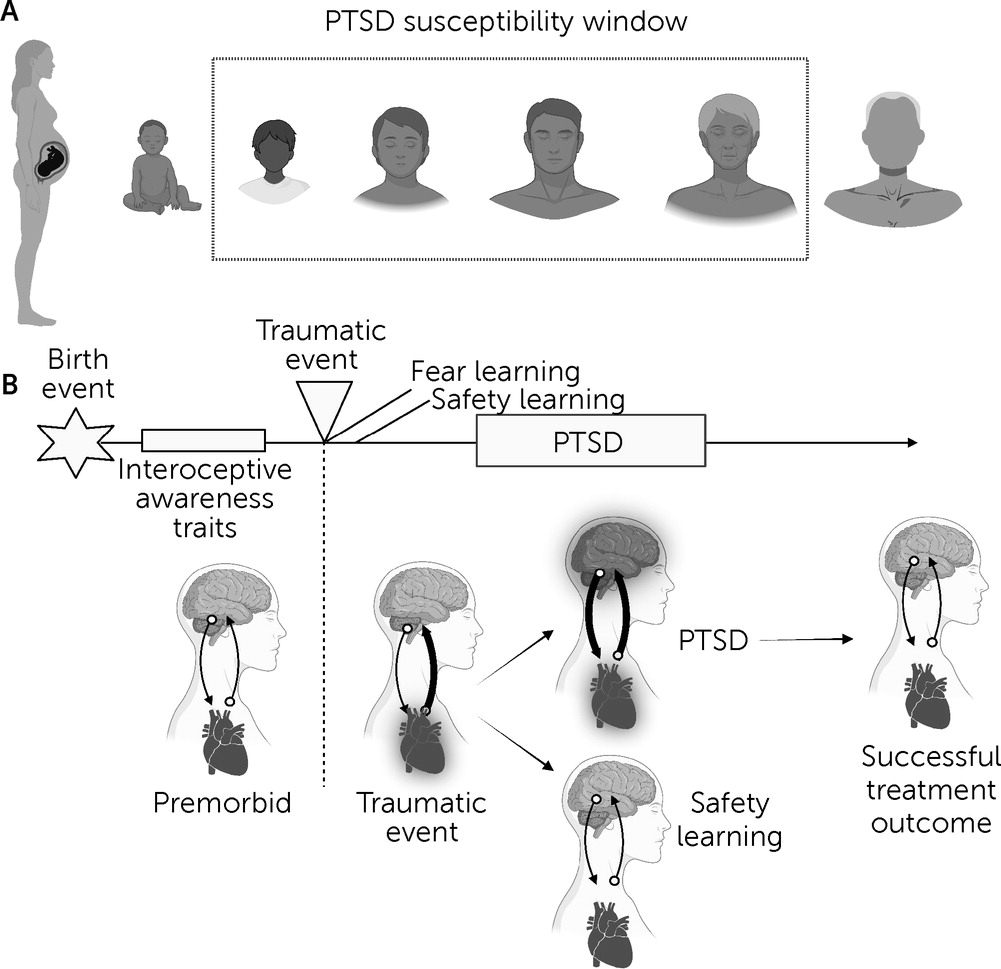
PTSD differs from other psychiatric conditions in that it is a temporally specific disorder triggered by the experience of a traumatic or life-threatening event that elicits heightened physiological arousal and fear. PTSD can follow traumatic events that occur at any point across the human life span, although the temporal susceptibility window typically starts in infancy, and the greatest sensitivity occurs from early childhood through middle adulthood (
Figure 1A) (
5,
6). During the traumatic experience, external and internal environmental cues are encoded within the brain and integrated to form fear-related memories. Traumatic experiences are common, with estimates of 70% of civilians having experienced a criterion A event during their lifetime (
7). However, only a subset of these individuals (approximately 6% of the general population) (
8) will proceed to meet criteria for PTSD. In adaptive trauma recovery, the associations built between threat and neutral signals at the time of trauma exposure are not reinforced, and the symptoms dissipate with time. However, in PTSD, these associations persist even when a threat is no longer present, and the symptoms are reinforced and strengthened through the avoidance of traumatic memories and trauma reminders.
Fear learning models (e.g., fear conditioning, fear extinction, fear reinstatement) are central to the leading theories regarding development, maintenance, and treatment of PTSD. However, these models are often considered separately from the role of internal physiological sensations in the affective processing of arousal and fear signals, representing a key gap. In this review, we propose that interoception, the process of sensing, interpreting, and integrating internal body signals, can offer valuable insight into the relationship between the experience of trauma and the fear learning processes that lead to PTSD. We start by reviewing the basic processes of fear learning and then consider interoception in relation to PTSD. We then focus our discussion on how interoception may intersect with current theoretical models of fear learning, beginning with the initial response to a traumatic event. We continue with a discussion of the effects of interoceptive signals on fear learning, extinction, and context processing. Finally, we identify avenues for further empirical and clinical research on interoception and its associated effects on fear learning among individuals with PTSD.
Fear Learning and PTSD: The Dominant Model
The processes considered most central to the development and maintenance of threat-related associations in PTSD include fear conditioning (
9,
10) and fear extinction learning (
11), respectively. Fear conditioning involves a process of learning to form associations between a neutral, conditioned stimulus (CS) and an unconditioned stimulus (US) that elicits an unconditioned response. In experimental paradigms, the repeated pairing of a CS with a US builds an association whereby the CS alone comes to elicit the unconditioned response, which is then called the conditioned response. A real-world example of conditioning could be the scent of a perpetrator’s cologne (i.e., CS) following a sexual assault (i.e., US) that may elicit a conditioned response (e.g., elevated heart rate, shallow breathing) when encountered in a safe setting. A life-threatening traumatic experience is usually considered a US that can become paired with various environmental and neurophysiological cues and contexts associated (or temporally bound) with the traumatic event. Because of the salience and intensity of a traumatic event, repeated pairing is not necessary to induce or condition a sustained fear response; PTSD is often observed after one traumatic event (e.g., a car crash or a sexual assault), as well as during situations in which the trauma has been repeatedly experienced over time (e.g., repeated combat-related exposure or sustained childhood abuse). Chronic stress from repeated traumatic experiences further reinforces fear conditioning through multiple pairings of the CS and US (
12). Individuals with PTSD may also exhibit a generalization of their fear response to stimuli distinct from, but similar to, a CS (e.g., fear of a grizzly bear following an attack extending to dogs or other large animals; fear of any people who share the physical attributes of a perpetrator of assault) (
13).
Fear extinction learning is a normative process that follows fear conditioning. It occurs when the CS is no longer reinforced by presence of the threat (US), and a new association between the CS and the experience of safety is formed. Given the sustained fear that characterizes PTSD, it has often been conceived as an inability to extinguish one’s fear after a trauma has ended, and deficits in laboratory-based extinction learning have been well-documented among individuals with PTSD. Compared with healthy trauma-exposed individuals, persons with PTSD frequently exhibit sustained fear responses (
14), or increased fear reinstatement (i.e., return of conditioned response), when a CS is presented after a delay following extinction learning (
15).
Context-dependent factors are thought to influence the acquisition and extinction of fear responses to specific stimuli or cues. Although conditioned cues predict the onset of a US, context is comprised of multiple internal and external factors (e.g., spatial, temporal, interoceptive, cognitive, social, and cultural) that convey information, which can influence the learning of associations about a CS (
16). To elaborate, the same cue may be differentially associated with threat or safety on the basis of contextual information (e.g., a lion in the wild may be associated with threat, whereas a lion in the zoo may be associated with safety). Deficits in context processing have been documented among individuals with PTSD (
17). In laboratory-based contextual fear paradigms, individuals with PTSD have shown persisting conditioned responses during extinction learning (
18) when a CS is presented in a safe context during extinction recall (
19). An example of context generalization is demonstrated when a combat veteran continues to display fear responses to cues previously associated with threat (e.g., rubble on the side of the road indicating presence of an improvised explosive device in a combat zone) when the cue is encountered in a safe context (e.g., rubble on the side of the road indicating presence of a construction site in the veteran’s neighborhood).
The neural mechanisms of exteroceptive fear conditioning are well established. During exteroceptive fear learning, multimodal perceptual inputs are relayed from the thalamus to the amygdala (
20). Neural associations are formed via the repeated activation and strengthening of synapses in the basolateral complex of the amygdala, and expression of fear is modulated by connections between the basolateral complex and regions of the prefrontal cortex (PFC) (
21). Contextual information is communicated through hippocampal inputs to the amygdala. The amygdala subsequently relays signals back to relevant body systems via the periaqueductal gray (PAG) (
22). The PAG orchestrates a cascade of innate and learned physiological responses involving autonomic (i.e., heart rate, respiratory, skin conductance) and endocrine (e.g., blood pressure, adrenergic-cortisol) responses (
23). The PAG is also thought to be involved in subsequent behavioral responses to threat, with the dorsal PAG involved in escape and contextual fear learning, and the ventrolateral PAG involved in freezing behavior or immobility (
24,
25). Bidirectional connectivity between the PFC and the basolateral amygdala is also thought to be central to inhibition of fear (i.e., fear extinction), leading to a reduction in the conditioned fear response via regulation of central amygdala reactivity (
26).
Previous research indicates that although the neural function underlying fear acquisition is similar for individuals with and without PTSD, there are differences observed in fear and memory circuity during extinction learning (
17,
19). Successful fear extinction following fear conditioning relies on hippocampal-dependent memory and has been associated with inhibition of fear expression via the ventromedial PFC (vmPFC) (
26,
27). Individuals with PTSD, compared with those without the condition, demonstrate decreased hippocampal activation, decreased within-network connectivity in the default mode network, and increased connectivity between the default mode and salience networks during fear extinction recall and fear renewal (
28).
By comparison, the role of interoception in fear learning and interoceptive fear conditioning has received limited study, despite the significance of interoceptive processing in the implementation of basic emotional states in foundational theories of emotion. For more than a century, these theories have focused on the interactions between subjective appraisal of emotion and interoceptive and exteroceptive sensory input (
29). The James-Lange theory of emotion (
30,
31) relied on the premise that afferent physiological and interoceptive signals constitute the drivers of affective experience. Rebutting this notion, the Cannon-Bard theory (
32,
33) posited that physiological and emotional experiences occur independently and are thus untethered to afferent interoceptive input. Attempting to integrate these perspectives, the Schachter-Singer theory (
34) proposed that the physiological and cognitive appraisal of emotional signals occurs in tandem, with attributions of emotional states driven by both physiological arousal and environmental context cues. Despite their considerable differences, which continue to be debated (
35), these theories underscore the firm recognition of the importance of interoceptive processing with respect to implementation of basic emotional states. However, only during the last several decades has a connection between interoceptive dysregulation and fear-related psychiatric disorders begun to be discussed (
36–
41). As we argue in the ensuing sections, investigating the role of interoception in fear learning and PTSD may provide essential insight into how differing profiles of PTSD symptoms are experienced by individuals.
Interoception: How the Nervous System Senses the Body’s Physiological Status
Interoception refers to the process by which the nervous system detects, interprets, and integrates information from the internal organs and tissues, serving as the basis for the conscious and unconscious experience of body sensations (
42). Interoception is essential for maintaining homeostasis, the reactive regulatory process responsible for maintaining the internal balance of bodily functions (
43), and for allostasis, the predictive regulatory process involved in adaptively controlling autonomic and endocrine responses in preparation for future internal and external stimuli (
44). Interoception also plays a critical role in the sensation and perception of internal body sensations, such as hunger, thirst, heart palpitations, breathing, and body temperature (among others); in the regulation of emotional and social behavior; and in the development of emotions and conscious higher-order states, including self-awareness, empathy, and self-regulation (
45). Interoception involves a network of neural pathways and brain regions that receive and process information from different organs and body systems. This network includes afferent fibers from sensory receptors located in peripheral organs, such as the heart, lungs, gut, and skin, which transmit signals to the spinal cord, brainstem, hypothalamus and thalamus, and then to higher cortical areas, such as the insular cortex, anterior cingulate cortex (ACC), and PFC (
46).
The insular cortex is a key brain region for interoception, as it receives and integrates information from many different body systems and is involved in generating subjective feelings of body states, such as hunger, thirst, pain, heartbeat sensations, and emotional arousal (
47,
48). The ACC is also involved in monitoring and regulating body states; it is reciprocally connected to the insula and has a role in the emotional and cognitive evaluation of interoceptive sensations (
49,
50). The PFC is also involved in the conscious processing and modulation of interoceptive information, enabling individuals to monitor and potentially control their body responses, such as heart rate and breathing, and to use this information to adapt to environmental demands and social contexts (
46,
51). In summary, interoception constitutes the nervous system’s monitoring of the internal state of the body, involving a complex network of neural pathways and brain regions that detect, interpret, and integrate information from different body systems, generating subjective feelings of body sensations, and enabling individuals to adapt to environmental demands and social contexts.
Interoception and PTSD: Empirical Evidence and Theoretical Models
Interoceptive dysregulation has been associated with a range of psychiatric disorders, such as anxiety, depression, eating disorders, schizophrenia, autism, somatic symptom disorders, and PTSD (
52,
53). Unlike these other psychiatric disorders, PTSD is triggered following the experience of a traumatic or life-threatening event. These events kickstart complex communication between the brain and body, particularly between neural circuits focused on processing interoceptive signals and those focused on processing exteroceptive cues and environmental contexts specific to the trauma. Studies have demonstrated that childhood traumatic events, early life adversity, and sexual trauma are associated with lower subjective interoceptive accuracy (
54), increased interoceptive accuracy on a heartbeat perception task (
55), and greater dissociative symptoms (
56). However, these recent findings are few in number, suggesting that substantial knowledge gaps remain.
Kearney and Lanius (
57) recently introduced a theoretical model to describe how PTSD disrupts the hierarchical processing of brain and body signals, creating a distorted sense of self. They highlight that, during exposure to a traumatic event, individuals may exhibit or attend to different internal responses and focus more or less on their physical sensations and ongoing cognitions. The model suggests that when traumatic events disrupt the brainstem-level processing of physical sensations (i.e., at the level of the PAG), there are downstream breakdowns in the expression and subsequent integration of body signals and upstream exacerbations of arousal and affective signaling that can include numbing and dissociative responses. These signaling disruptions alter the basic sense of self as it relates to the experienced environment, which influences how limbic and other intrinsic brain networks (such as the default mode network) regulate self-referential processing and embodied self-consciousness. Neocortical modulations of this distorted self-representation perpetuate a less reflective, less embodied, and subordinate sense of self. This model of PTSD suggests that the sensing and processing of interoceptive sensations is in fact fundamental to the development of the disorder and could help to explain certain states of disembodiment, such as dissociation, which involves detachment from one’s sense of self (i.e., depersonalization), disconnection from surroundings or reality (i.e., derealization), or blunted emotional responsivity (i.e., numbing) following a trauma. Although the model focuses on dissociative symptoms, it also has relevance for nondissociative presentations of PTSD. Specifically, nondissociative presentations of PTSD have been characterized by exaggerated threat responses thought to relate to diminished top-down prefrontal control over the amygdala (
58), whereas dissociative presentations of PTSD have been conceptualized as involving an overregulation of the amygdala, with increased activation in cortical regions (i.e., dorsolateral PFC, ACC, insula) involved in attentional control and interoception (
58), and increased functional connectivity between these regions and the amygdala (
59). As such, the integration of interoceptive, exteroceptive, affective, and self-referential processing likely lies on a continuum and contributes broadly to fear learning and extinction and the symptoms experienced by individuals with PTSD.
Interoception in Context Processing: Neural Mechanisms and Behavioral Implications
From an interoceptive standpoint, the body may be viewed as a lens through which the world is experienced and remembered. Contextual factors surrounding the occurrence of conditioned cues can thus meaningfully inform whether these cues may or may not be predictive of threat, and these factors may be extended to include the state of the body at the time of the traumatic event. Individuals with PTSD have shown deficits in contextual processing (
16,
17), contributing to impaired extinction learning when CSs are presented in safe contexts. Studies examining context processing in PTSD typically focus on external cues and contexts (e.g., rooms paired with colored lights as the CS) (
19). However, interoceptive body sensations may also serve as negative internal contexts, conveying information to the nervous system that could reinforce threat conditioning to external cues or prevent safety learning through extinction. Conversely, external contexts (i.e., spatial or temporal) could inform when bodily symptoms become predictive of negative outcomes (
Figure 3). For example, increased cardiovascular arousal in the gym may not be predictive of negative outcomes. However, the same responses when walking in a dark alley may be a response to help facilitate hypervigilance to potential threats. In turn, if one’s physiological arousal is blunted during a potential fear-related context (e.g., because of alcohol use or other drugs), this blunting may contribute to reductions in predicted negative outcomes to that context. The potentially important role of interoceptive signaling in fear learning was elegantly demonstrated in a study (
87) reporting higher SCR responses when exteroceptive cues were paired with aversive shocks during cardiac systole compared with diastole. This finding suggests that differences in physiological activity during safe and threatening contexts, and awareness of these differences, may be useful for individuals when discriminating between safe and threatening contexts.
Few studies have used interoceptive cues in contextual conditioning paradigms among humans, and to our knowledge, no studies have been conducted among individuals with PTSD. In a sample of healthy participants (
88), benign visceral sensations (CS) paired with painful stimulation (US) in a context connoting threat were associated with increased activation in the vmPFC, amygdala, and hippocampus. In the context connoting safety, greater hippocampal responsivity to the CS was related to less negative valence ratings of the context and greater expectancy ratings. Another study (
89) demonstrated that suffocation fear moderated the persistence of startle eye blink responses to mild dyspnea (CS, induced via a small inspiratory breathing resistive load) that preceded a severe dyspnea (US, induced via maximally tolerable breathing load), in a context where presence of the US was unpredictable. Contextual conditioning was not reflected by explicit arousal and valence ratings nor was there evidence of declarative memories for the various experimental contingencies. In addition to providing evidence that context-specific conditioning to benign interoceptive cues can recruit fear neurocircuitry, these studies have illustrated how there can be discrepancies between conditioning and conscious awareness via verbal report.
Animal models have further corroborated the role of interoception in contextual processing. One study conducted with rats (
90) reported evidence of context-dependent conditioning, with differences in acute startle responses to interoceptive CSs but not to exteroceptive CSs, in threatening compared with safe contexts. Another study (
91) demonstrated disrupted hippocampal engagement and extinction learning in mice when internal, rather than external, context was manipulated through ethanol administration during fear acquisition. Further support for interoceptive involvement in context processing was evidenced by a study (
92) in which inactivation of the insular cortex in mice contributed to reduced freezing behavior in response to predator odor, as well as reduced contextual learning.
Situating Interoception in PTSD Treatment and Outcomes
Given the varied role that interoceptive processing likely plays in fear learning, there are numerous ways in which one could imagine targeting interoception when attempting to improve behavioral and psychopharmacological treatment outcomes for patients with PTSD. These possibilities include facilitating extinction to interoceptive experiences associated with the trauma via exposure-based therapy, to break the cycle of avoidance of these signals and interrupt further higher-order conditioning to interoceptive cues; boosting the interoceptive signal-to-noise ratio in an effort to increase interoceptive awareness, which could potentially change the specificity of trauma memories or higher-order conditioning, increase confidence in one’s own internal sensations and predictive cues, and enhance awareness of and learning from prediction errors; and directly modulating interoceptive signaling, via pharmacologic interventions, to strengthen or weaken the salience of these cues and their associated predicted negative outcomes to modulate fear learning.
Evidence-based behavioral interventions (e.g., prolonged exposure therapy) use inhibitory learning to target avoidance surrounding trauma reminders and memories through repeated exposure. These interventions often target exteroceptive trauma reminders (i.e., through in vivo exposures), whereas interoceptive exposures specifically focused on eliciting feared internal sensations (e.g., running in place to increase heart rate) are often reserved primarily for panic disorder (
93). However, preliminary evidence has suggested that interoceptive exposure therapies for PTSD are effective in reducing trauma-related symptoms (
94–
96). Interoceptive exposures for PTSD often involve inducing the interoceptive sensations (e.g., elevated heart rate, shallow breathing) that were experienced during the traumatic event, but in a safe context (i.e., therapy setting), to target expectancy of predicted negative outcomes (e.g., experiencing further trauma or expectancies about not being able to tolerate the distress). Additionally, interoceptive exposures for PTSD may be combined with imaginal or in vivo exposures to optimize inhibitory learning (
97). Inducing interoceptive sensations may elicit memories of the traumatic event that could be elaborated on through a combined imaginal exposure. Exposure to combinations of interoceptive and external contextual cues, in tandem, may also maximize safety learning by enhancing prediction error and awareness of these errors.
Given previous evidence that sensitivity to interoceptive state may influence extinction learning and serve as a meaningful internal context that could influence outcomes, it has been argued that behavioral interoceptive interventions focused on enhancing interoceptive awareness could enhance learning (
53). Several interventions may be useful for targeting enhanced interoceptive awareness and regulation in the treatment of PTSD. For example, there is evidence that mindfulness-based and mind-body interventions (e.g., yoga, tai chi) may be effective for some individuals with PTSD (
98,
99). Potential mechanisms for these benefits include increased attentional control, openness to experience, nonjudgmental acceptance of cognitions, as well as greater connection and awareness of interoceptive sensations. Neuroimaging research to date suggests that mindfulness may exert its effects by influencing brain activation and connectivity within and across the default mode network (e.g., posterior cingulate cortex, medial PFC), salience network (e.g., insula cortex, dorsal ACC), and executive network (e.g., lateral PFC) (
98). In addition, there is some evidence that mindfulness interventions may increase extinction retention (
100) and have an impact on hippocampal-dependent contextual retrieval (
101). Floatation-Reduced Environmental Stimulation Therapy is an intervention that increases interoceptive awareness in part by attenuating exteroceptive sensory input (
102,
103), but thus far no studies have focused exclusively on individuals with PTSD. Further research is warranted to identify whether interventions directly targeting interoceptive awareness may be beneficial for individuals with PTSD, to identify strategies for optimizing the impact of mind-body interventions on interoceptive awareness, and to delineate how targeting interoceptive awareness may affect specific fear learning processes.
Pharmacologic manipulations may be used to directly enhance or dampen physiological responsivity in ways that could affect interoceptive awareness, fear learning, or PTSD symptoms. Prior research (
104) has also suggested that extinction learning during behavioral interventions may be enhanced through combination with pharmacological approaches. For example, propranolol has been paired with trauma recollections as a way of disrupting the reconsolidation of trauma memories by reducing sympathetic arousal and has been found in some studies to lead to a reduction in PTSD symptoms although the findings have been mixed (
105), and benefits may be more likely if propranolol is combined with exposure therapy soon after a traumatic event (
106). Interestingly, a review (
107) of pretreatment biomarkers for PTSD found that greater heart rate reactivity (e.g., change in heart rate in response to external stressor), but not SCR, to trauma reminders at the onset of PTSD treatment predicted posttreatment symptom reduction. The beneficial effects observed with propranolol may in part be due to increases in prediction error when the interoceptive signals one expects to experience during trauma recall are not experienced. Pharmacologic manipulations that enhance sympathetic arousal (e.g., isoproterenol) have been used as a way of assessing interoceptive awareness across states of homeostatic perturbation (
51,
108). This method may potentially also be used to train interoceptive awareness or to enhance interoceptive exposure-based therapy. In an intriguing pilot study (
109), the application of methylphenidate, a central nervous system stimulant that augments cerebral dopaminergic and noradrenergic function, was well tolerated, and found to reduce PTSD symptoms of reexperiencing, avoidance, and hyperarousal. Although mechanistically unclear, the possibility that modulating dopaminergic and noradrenergic arousal might help in improving interoceptive awareness, by increasing the interoceptive signal-to-noise ratio and by reducing the unpredictability of the internal world, appears worthy of follow-up. Overall, further work is needed to identify how interoceptive perturbations may influence fear learning processes in PTSD and to understand how to optimize the use of interoceptive processing to enhance prediction error or other fear extinction processes.