Depression in Polycystic Ovary Syndrome: Focusing on Pathogenesis and Treatment
Abstract
Introduction
Depression in PCOS
Epidemiology
| Type | Country and case inclusion period | Prevalence (%) | Groups (number of subjects) | Treatment | Treatment length | Assessment scales | Results | References |
|---|---|---|---|---|---|---|---|---|
| Summary of studies indicating prevalence of depression in PCOS by geographic region | United States, 2005–2008 | 64.1% | PCOS with depression (n = 75), PCOS without depression (n = 42) | / | 35 months | PHQ-9 | The prevalence of depressive disorders among women with PCOS was 64.1% | (8) |
| | United States, 1985–1986 | 36% | No PCOS (n = 1,044), PCOS (n =83) | / | 12 months | CESD | CES-D scores were higher among women with PCOS, and black women experienced higher depression burden than white women | (9) |
| | Australia, 1973–1978 | 27.3% | PCOS (n = 478), non-PCOS (n = 8,134) | / | 60 months | CESD-10 | Women with PCOS, reported higher prevalence of depression than women without PCOS (27.3 vs. 18.8%) | (10) |
| | Korean, 2007–2010 | 15.35% | PCOS (n = 26,251), Non PCOS (n = 131,480) | / | 36 months | / | The risk of developing depressionin women with PCOS was higher compared to women without PCOS | (11) |
| | Syria and Jordan, 2017 | 83% in Syria and 65% in Jordan | Syria (active, n = 30 vs. control, n = 30), Jordan (active, n = 30 vs. control, n = 28) | / | 5 months | Beck depression inventory | Syria and Jordan highlighted a high prevalence of depression (Syria = 83% vs. Jordan = 65%) | (12) |
| Randomized controlled trials assessing the effect of PCOS-related treatments on depression in women with PCOS | Netherlands, 2010–2016 | / | CAU (n = 60). CBTLS (n = 63) CBTLS+SMS (n = 60) | Cognitive behavioral lifestyle sessions combined with a healthy diet and physical therapy | 12 months | BDI-II, RSES, FNAES | A three-component lifestyle intervention based on CBT could improve depression in women with PCOS | (13) |
| | United States, 2013–2015 | / | CBT+LS (n = 20), LS (n = 13) | Cognitive-behavioral therapy (CBT) and lifestyle modification (LS) | 16 weeks | CESD, STAI | CBT+LS significantly improved depressive symptoms in women with PCOS compared with LS alone | (14) |
| | China, 2018–2019 | / | Intervention group (n = 61), control group (n = 61) | Transtheoretical model-based mobile health application intervention program | 12 months | SAS, SDS | TTM-based mobile health application program can decrease depression in patients with PCOS | (15) |
| | Australia, not mentioned | / | HPLC: (n = 14); LPHC: (n = 14) | High-protein, low-carbohydrate diet (HPLC) | 16 weeks | HADS and the Rosenberg Self Esteem Scale | The HPLC diet was associated with significant reduction in depression | (16) |
| | Brazil, 2014–2016 | / | CAT (n = 23), IAT (n = 22), CG (n = 24) | Continuous and intermittent aerobic physical training | 16 weeks | HADS | Both CAT and IAT groups had significant reductions in depression scores | (17) |
| | China, 2016–2019 | / | A (n = 20), LS (n = 20). | Acupuncture | 4 months | SAS, SDS | Acupuncture can effectively relieve depression in patients with PCOS, and the mechanism may be related to the regulation of serum β-endorphin and androgen | (18) |
| | Swedish, 2005–2008 | / | Acupuncture (n = 28); exercise (n = 29); control (n = 15) | Acupuncture | 16 weeks | MADRS-S, BSA-S | Acupuncture can lead to a modest improvement in depression scores in women with PCOS | (19) |
| | China, 2012–2016 | / | Acupuncture group (n = 27), sham acupuncture group (n = 27) | Acupuncture | 16 weeks | Zung-SAS and Zung-SDS | Acupuncture can influence serum levels of NE and 5-HT, improving symptoms of depression in PCOS patients | (20) |
| | United States, 2008–2014 | / | OCP group (n = 45), LS group (n = 44), combined group (n = 43) | Oral contraceptive pills (OCPs; ethinyl estradiol/norethindrone acetate) | 16 weeks | Positive screens on the Prime-MD | OCPs result in significant improvements in depressive symptoms | (21) |
| | Athens, 2012–2013 | / | Intervention group (n = 23), control group (n = 15) | Mindfulness stress management program | 8 weeks | DASS 21 | Mindfulness techniques ameliorate stress, anxiety, depression and the quality of life in women with PCOS | (22) |
| | Danish, 2014–2016 | / | MI+ SA (n = 19), SA (n = 18) | Motivational interviewing | 6 weeks | WHO-5 and MDI | Motivational interviewing can significantly improve depression scores | (23) |
| | Germany, 2011–2012 | / | Pioglitazone (n = 20), metformin (n = 20) | Pioglitazone | 6 weeks | HDR-17 | Pioglitazone improves depression with mechanisms largely unrelated to its insulin-sensitizing action | (24) |
| | China, 2016–2018 | / | PM (n = 28), M (n = 26), placebo (n = 21) | Pioglitazone metformin complex preparation (PM) | 12 weeks | SCL-90-R | Pioglitazone metformin alleviates depression via inhibiting NLRP3 inflammasome | (25) |
Pathogenesis of PCOS
Pathogenesis of Depression
Pathogenesis of Depression in PCOS
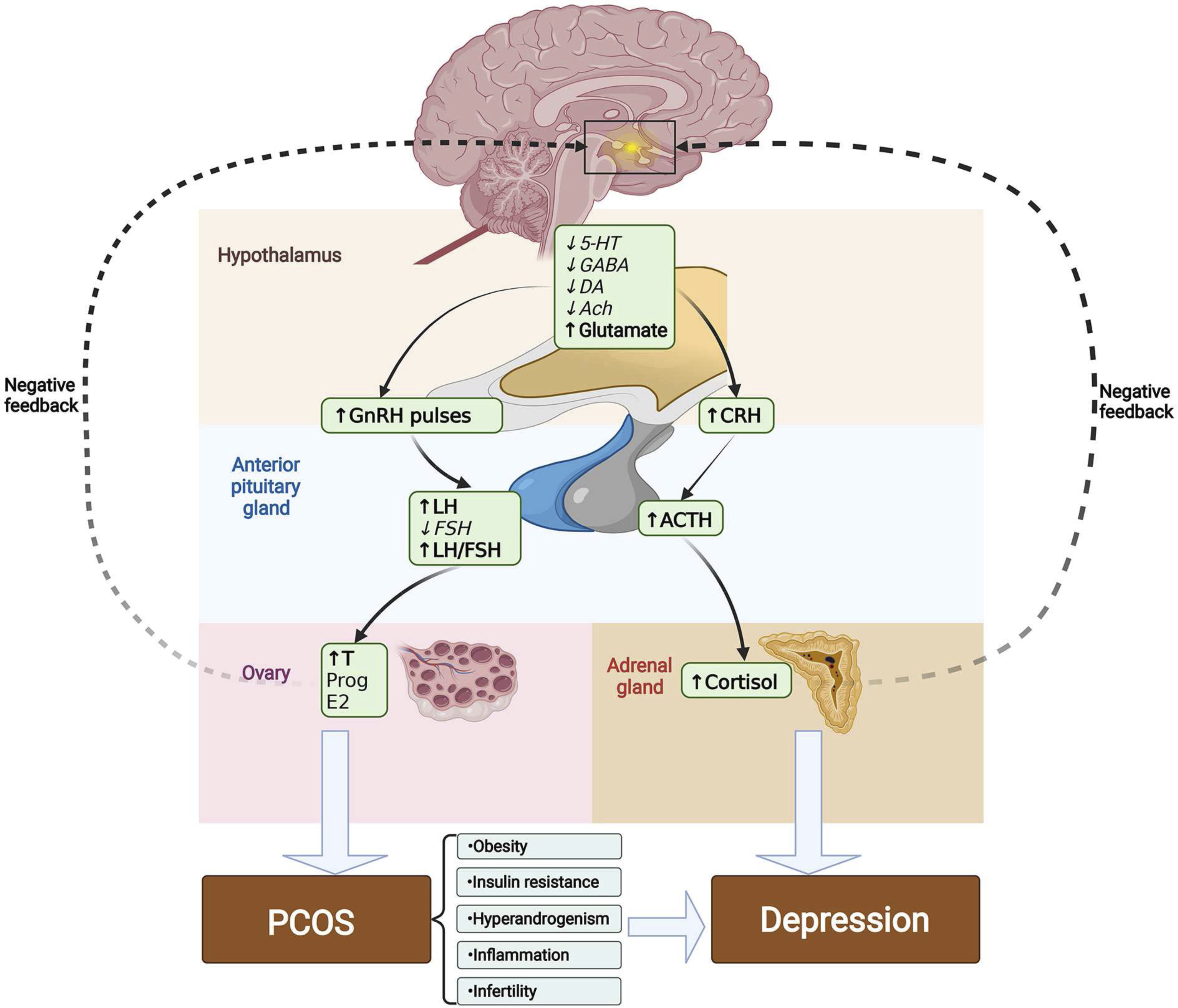
Obesity
Insulin Resistance
Hyperandrogenism
Inflammation
Infertility
Treatment
Lifestyle Intervention
Acupuncture
Oral Contraceptive Pills
Psychological Intervention
Insulin-Sensitizer
Summary and Outlook
Footnotes
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

