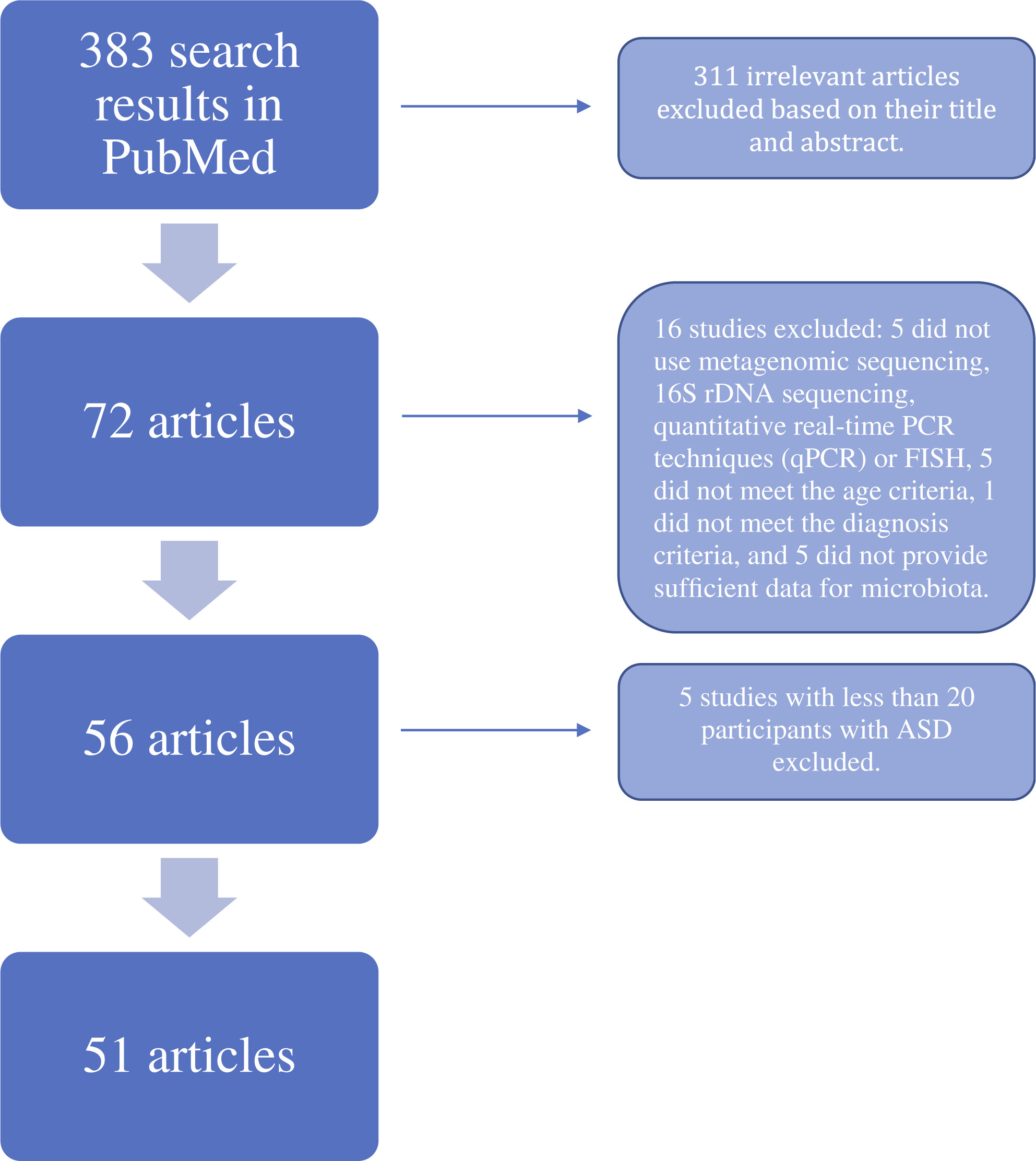
Discussion
The aim of this study was to perform a systematic review of the current knowledge on ASD and the gut microbiota in children. The results show that the gut microbiota of children with ASD is altered compared to one of neurotypically developed children. Most studies in this review found significant differences between microbial communities, while there was notable variation in results regarding diversity indices or taxonomic level abundance. The most coherent results regarding taxa level differences in ASD children’s gut microbiota compared to controls were in Proteobacteria, Actinobacteria and Sutterella levels, that all seemed to be increased in ASD.
Beta diversity describes the dissimilarity between overall microbial communities. The total of 26 studies in this review reported that the gut microbiome of ASD children was significantly different compared to the control samples, while two studies with no significant difference. Beta diversity was also found to be affected by dietary habits
38 and geographical location.
46 However, results regarding alpha diversity were more contradictory. Most studies did not specify the alpha diversity index; therefore, it remains unclear whether the difference in the diversity is explained by richness of different species or a dominance in a specific species. Liu et al. made similar remarks indicating that while it seems that the gut microbiota is indeed altered in ASD, the significance of diversity in a single sample remains unclear.
59Apart from three studies, all studies that found no significant difference in the alpha diversity had acknowledged the diet of participants. In many of those studies, participants with restricted diets were excluded or all participants were given a similar diet. Diet is known to directly alter the composition of gut microbiota,
60 therefore, homogenous diets often observed in ASD children could result in less intra-individual but more inter-individual variety in gut microbiota as well. Three out of five studies that had results indicating that participants with ASD had higher diversity did not report the diets of participants, while most studies with results indicating that ASD children had lower alpha diversity had reported the diets. Berding and Donovan found no difference in alpha diversity when comparing the gut microbiota of ASD children to healthy controls but found that dietary patterns were associated with specific microbial profiles.
31 Children who consumed more vegetables, legumes, nuts and seeds, fruit alongside with refined carbohydrates and starchy vegetables had microbiota profiles characterized by lower abundance if
Enterobacteriacae, Lactococcus, Roseburia, Leuconostoc and
Ruminococcus, while the gut microbiota of children who consumed less of those foods had higher levels of multiple species, including
Barnesiellacaea and
Alistipes. More restricted diet could, therefore, result in increased abundance of certain species. However, in the same study, diet and microbiota did not predict social deficit scores. In a recent large autism stool metagenomics study by Yap et al., restricted dietary preferences seemed to result in less diverse gut microbiota and additionally, looser stool consistency.
52 They did not find evidence for gut microbiota directly contributing to the etiopathogenesis of ASD. There are also other factors, such as age and type of delivery, that could affect the composition of the gut microbiota.
42 Of note, as there was great variety in how restricted diets were defined and assessed in the reviewed studies, heterogeneity of diets is likely to affect results. Another source of uncertainty is how much these different diets are associated with GI problems and altered gut microbiota composition. We noted no clear association between study characteristics and whether differences in alpha diversity were reported. These results show that instead of solely focusing on the microbial differences, more research is needed to understand the connection between diet, gut microbiota, and ASD in children, and furthermore, the factors affecting microbial diversity.
Williams et al., Zhang et al., Zhai et al., and Chen, Shi et al. had aligned results indicating that
Sutterella is more abundant in children with ASD.
11,16,23,54 Williams et al. discovered that
Sutterella could be found in the gut microbiota of more than half of children with ASD, while none of the controls had it. The above-mentioned studies included both subjects with and without GI symptoms. These findings could indicate that
Sutterella may be associated with ASD regardless of GI symptoms.
Five studies confirmed that Proteobacteria was found in higher abundance in children with ASD. In only one study Proteobacteria levels were decreased. Li et al. found that also the mothers of children with ASD had a higher abundance of Proteobacteria, which could be explained by dietary habits and other environmental factors but also with congenital factors that alter the gut microbiota of children.
12 In a study by Plaza-Diaz et al., participants were divided into mental regression and non-mental regression groups based on their ADI-R score. They found that Proteobacteria were more abundant in ASD children with mental regression compared to those with ASD and no mental regression.
8 There is evidence for Proteobacteria being associated with inflammatory processes in the GI tract.
61,62 These results could indicate that Proteobacteria, that include many potentia1 pathogens such as
Escherichia, Vibrio, Helicobacter, Salmonella and
Yersinia could affect not only the gastrointestinal tract but also neuropsychiatric function of children.
While there seems to be some features of gut microbial composition that are typical to children with ASD, different clinical pictures of ASD seem to be characterized by differences in the gut microbiota as well.
46,53 Ding et al. reported that the severity of ASD symptoms seems to affect the composition of the gut microbiota.
42 More severe phenotype of ASD was characterized with higher levels of
Lachnospiraceae and
Erysipelotrichaceae and lower levels of
Faecalibacterium. The observed dose–response in ASD severity and
Lachnospiraceae abundance might indicate a causal relationship. The members of
Lachnospiraceae family have yielded mixed associations with pathology, and likewise, the were discrepancy in our review regarding associations between
Lachnospiraceae abundances and ASD.
63 Although the members of
Lachnospiraceae family are known SCFA-producers, the differences in microbial networks and cross-feeding patterns may result in different physiological effects. Additionally, it has been suggested that the severity of ASD symptoms could be connected to specific gut microbial characteristics.
46,53 In a study by Hua et al. children with ASD were divided into two groups based on whether they had a comorbid sleep disorder,
41 and those who suffered from sleep disorder had also more severe ASD symptoms. It was discovered that the microbial profiles of the two groups were significantly distinct. Children with ASD who also suffered from sleep disorders had higher abundance and richness of gut microbiota. Nevertheless, while some of the clinical features of ASD seem to be associated with the gut microbial composition, a correlation between the two is hard to confirm.
35Comorbid gastrointestinal symptoms could be characterized by a distinct microbial profile as well.
20 While some studies had excluded all participant with any GI symptoms, others only included those with GI symptoms to both study and control group. This variation could partially explain the heterogeneity of results, although no clear association with participant's GI symptoms seemed to explain the differences in findings. Most studies had not mentioned if the patients were screened for other neuropsychiatric disorders, which could confound the results. Possible microbial differences can also be explained by behavior typical to ASD, such as restrictive eating patterns and potential therapies.
Four studies reported higher levels of Actinobacteria in children when compared to controls. Only one study reported decreased levels of Actinobacteria. In a recent meta-analysis by Chavira et al., higher levels of Actinobacteria in children with ASD was one of the most coherent results.
64 It was speculated whether this could indicate that the gut microbiota of children with ASD is underdeveloped, as Actinobacteria is more abundant in younger children. Three trials that included an intervention had interesting results regarding changes in behavioral symptoms among children with ASD. In the study conducted by Grimaldi et al. children with both restricted diet and B-GOS intervention had higher levels of
B. longum alongside with improved ATEC scores indicating improvements in sociability.
10 Consistently, Wang et al. discovered that
Bifidobacteriales and
B. longum were significantly increased after probiotics and fructo-oligosaccharide supplementation.
27 Both ATEC and 6-GSI score were decreased indicating improvements in autistic and GI symptoms. Shaabam et al. had similar results: after probiotic supplementation, children with ASD had an increase in
Bifidobacteria abundance as well as improvements in ATEC scores.
14 Meanwhile, Ahmed et al. discovered that the only difference in the gut microbiota of ASD children compared to their healthy siblings was that the siblings had higher levels of
Bifidobacteria, which could act as a protecting factor.
35 B. longum has been previously connected to reduced stress levels,
65 which indicates that it has a significant role in the gut-brain-axis function. While this review shows that the abundance of
Bifidobacteria seems to vary in children with ASD (
Table 2),
Bifidobacteria and especially
B. longum could be targeted more specifically in probiotic treatment of ASD children in the future, although as concluded in a systematic review by Ng et al., more research with standardized intervention regimen is needed to study the potential benefits of supplementation treatments.
66Some of the studies had also studied different microbial metabolites. These results give potentially more mechanistic insight to understanding the gut-brain-axis. In the current review, multiple studies discovered that SCFAs were associated with ASD. Studies that had looked at SCFA fecal concentrations, SCFA producing bacteria or genes coding SCFA producing enzymes all confirmed differences in children with ASD compared to controls. Differences in SCFA levels can be explained by abundance of certain SCFA producing bacterial taxa or the amount of carbohydrates in diet. Most studies that had reported SCFA levels had excluded participants with major dietary differences, indicating that there were differences in certain SCFA producing bacterial abundances. Two studies confirmed that there was indeed a connection between SCFA concentrations and levels of certain taxa. However, it should be noted that the methods used to detect functional markers varied between studies and was not considered in this review. Four studies found that lower level of butyrate was associated with ASD. However, other three studies found no significant difference in fecal butyrate levels. Butyrate is a SCFA that has been mostly associated with maintaining gut health but there has been growing interest of the neuromodulative effects of butyrate.
67 Butyrate has been associated with attenuating ASD behavior in animal models
68 and it was discovered that it could affect genes linked to behavior and cognition via energy metabolism by Rose et al.
69 In one study, more severe ASD symptoms were connected to microbial taxa involved in butyrate metabolism. Also, acetic acid levels seemed to be lower in ASD. The results of this review further support the idea of butyrate and other SCFAs playing a role in developing both gastrointestinal and behavioral symptoms in ASD, although the number of studies with fecal metabolomics data in child ASD research is still scarce.
It has been speculated whether specific microbial differences could be used as biomarkers for ASD. While this review confirms that there seems to be multiple differences in the gut microbiota of ASD children compared to healthy controls, the specific species that could be used as biomarkers remain controversial. There is significant variety in how the differences are classified, for example, whether the study has looked at phyla or genus level. Most of the studies included in this review used 16s rRNA sequencing to analyze the composition of the gut microbiota, but there were studies using
Clostridium perfringens targeted PCR, quantitative real-time PCR, shotgun metagenomics and FISH methods as well. Thereby, results that have been achieved using different methods cannot be fully compared. Almost all studies had used stool samples to analyze the gut microbiota. Using gut biopsies could provide more information on species that cannot be detected in stool samples. Furthermore, this review focused only on gut microbiota as this domain has been researched in the context of ASD sufficiently to conduct systematic literature review, but there is some research on other human microbiota as well, such as a study by Kong et al. discovering alterations in oral microbiota in ASD.
9 To develop a full picture of the connection between microbial changes and ASD, more research on non-gut microbiota could be beneficial.
Many studies in this review had a relatively small sample size, most including less than 100 participants. Additionally, in several studies used data was collected in one clinic or hospital. This review includes studies that have been conducted in different continents, including Asia, Europe, Africa, and Northern America, which can explain contradictory results, as the gut microbiota is likely to be affected by environmental and host genetic factors as well as cultural dietary characteristics. All these factors partly explain the complex results in this review. This review is only descriptive and narrative, and there are more advanced statistical methods available, as it was done in a large meta-analysis by West et al.
70 The inclusion of a wider range of databases than what was used in this review could provide more knowledge on the subject. Our findings complement those of the meta-analysis, since many datasets are not openly available in public repositories or shared upon request.
70 It could be beneficial to study comparative larger cohorts like this, including participants from different locations and utilize replication data sets. This could bring more epidemiological or population level insight on the subject.
All studies in this review had included more male than female participants, three of the studies including only male participants, which is explained by well-known differences in prevalence of ASD among boys and girls.
71 Thereby, it should be noted that the sex-ratio of children with diagnosed ASD affects participant recruitment and furthermore, results of a study, and the results might not be as representative when looking at girls with ASD. Also, the selection of controls varied in different studies. Some of the studies had used typically developed siblings as controls, while in other studies, controls were unrelated, which can influence the interpretation of results since the gut microbiota of neurotypical siblings is more similar to ASD group than to unrelated controls.
24,35 Nevertheless, most of the studies using siblings as controls did find significant differences between ASD children and their healthy siblings, and studies with both related and unrelated controls would give better insight in shared environmental and host genetic factors. This review highlights what is already known about the gut microbiota and ASD in children.