Parkinson's disease (PD) affects as many as 1 million people in the United States alone, with approximately 40,000 Americans diagnosed with the disease annually. The hallmark neuropathology of the disease is loss of dopaminergic nigrostriatal neurons and intracytoplasmic Lewy bodies in the substantia nigra and locus coeruleus.
1 The disease has a high degree of morbidity and is associated with significant use of nursing-home services, antidepressant and antipsychotic treatment, and overall reduced survival time, versus people without PD.
2 Deep brain stimulation (DBS) is an FDA-approved surgical treatment for management of advanced PD. As compared with medication management, DBS significantly improves motor symptoms and quality of life.
3–5 The subthalamic nucleus (STN) is used more commonly as the anatomic target for DBS, although a 24-month, randomized, blinded trial comparing STN and globus pallidus internus (GPi) stimulation showed similar motor function improvement.
6 However, there were several secondary outcomes suggesting differential impact on mood on the basis of anatomic targets. At study endpoint, the STN group, as compared with GPi, had a statistically significant higher score (p=0.02) on the Beck Depression Inventory (STN: 12.5 [standard deviation {SD}] 8.5 versus Gpi: 9.8 [7.3]). Subscales quantifying mood and emotional well-being on PD rating scales, including the United Parkinson's Disease Rating Scale (UPDRS) and Parkinson's Disease Questionnaire (PDQ–39) showed similar differences in STN versus Gpi.
6 In contrast, a recent 7-month, double-blind, randomized study comparing cognition and mood as primary outcome in STN and GPi groups (COMPARE Trial) reported no significant differences in mood and cognition outcomes between the two groups
7 in the optimal stimulation condition.
In a review of 82 studies that included almost 1,400 PD patients, STN DBS has been associated with new-onset psychiatric adverse events, including depression (8%), hypomania/mania (4%), anxiety (2%), and attempted suicide (0.4%);
8 although the sample is large, definitive conclusions are limited, given the lack of standardized assessment of psychiatric diagnosis and/or adverse events. Given the baseline estimate of PD comorbidity with psychiatric disorders, including depression (25%), psychotic syndromes (12.7%), sleep disturbances (49%), and anxiety (20%),
9 plus the additional risk associated with STN DBS, there is great need to understand the clinical correlates associated with STN DBS-induced psychiatric adverse events (PAEs). Given a recent report of voltage-dependent mania associated with STN DBS in a PD patient,
10 this literature review is focused on identifying underlying neurobiological mechanisms and clinical correlates of STN DBS-induced mania.
METHOD
We performed a structured MEDLINE (PubMed) search through December 2010, with keywords including subthalamic nucleus (STN), Parkinson's disease (PD), deep brain stimulation (DBS), mania, hypomania, and mood elevation. Seven published case reports,
11–15 two case series,
16,17 and one retrospective cohort study
18 with well-documented demographics, stimulation parameters, and details of psychiatric symptoms to confirm STN DBS-induced hypomania/mania were identified (total N: 17 patients).
L-dopa equivalent daily dose (LEDD) was computed with the following formula: total
L-dopa (regular
L-dopa dose × 1) + (
l-dopa controlled-release × 0.75) + (pramipexole dose × 67) + (ropinirole dose × 16.67) + (pergolide dose × 100) + (bromocriptine dose × 10), + [(
l-dopa +
L-dopa controlled-release × 0.75) × 0.25 if taking tolcapone {regardless of tolcapone dose}]).
19RESULTS
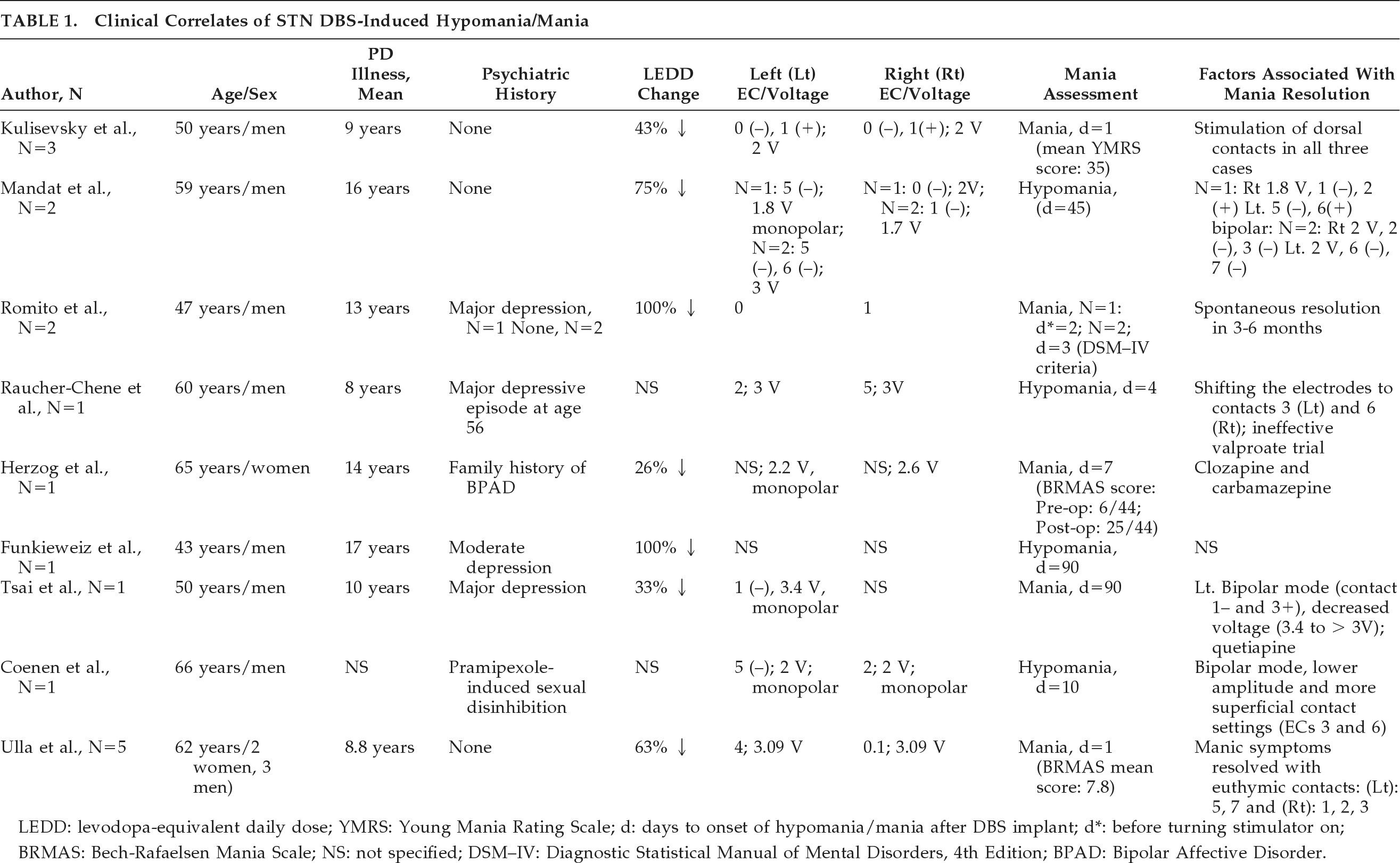
The clinical correlates associated with STN DBS-induced hypomania/mania can be subcategorized into electrode placement, stimulation parameters (unipolar versus bipolar stimulation and voltage), and neurosurgical (i.e., lesioning effect), neurological (age at onset of PD, LEDD change), and demographic factors (
Table 1).
In all, 14 of 17 patients (82%) developed STN DBS-induced hypomania/mania with ventromedial STN electrode placement.
12–18 This pattern was reproducible (i.e., induction of manic symptoms with ventral stimulation and resolution of manic symptoms with switch to dorsolateral simulation) in five patients.
17 Where reported, manic symptoms appeared to resolve in 12 of 14 patients (86%) after switching from ventromedial to dorsolateral STN; this occurred with an electrode configuration switch-alone in 9 of 12 patients (75%) or in combination with changes in stimulation parameters (i.e., voltage and stimulation mode) in 3 of 12 patients (25%).
In two case reports
13,18 both voltage >3 V and monopolar stimulation mode were associated with STN DBS-induced mania. Monopolar stimulation produces a current that runs in multiple parallel paths. This kind of stimulation may influence a larger volume of tissue, especially when the current density is relatively high. Bipolar stimulation produces a more concentrated electric field around the electrode pair.
20 Both increased voltage and unipolar stimulation can potentially result in enhanced current spread throughout the local tissue (relative to lower voltage or bipolar stimulation) and thus would be expected to influence a greater proportion of STN and nearby cells.
21 Lowering voltage, along with switching to bipolar stimulation mode, has been associated with manic symptom resolution.
13,18The clinical correlates associated with STN DBS-induced hypomania/mania in the immediate and early postoperative period may include localized edema, a partial lesioning effect due to the STN DBS implant,
11 as well as a possible add-on effect of STN DBS to the
L-dopa treatment.
5,18,22 The mean duration until onset of hypomania/mania in 17 patients was found to be 17.8 (30.6) days.
Among the demographic and neurological correlates, 14 of 17 patients (82%) with STN DBS-induced hypomania/mania were men (mean age: 55.6 [10.6] years), with relatively early onset of PD (N=13; mean age at PD onset: 43.6 [11.5] years) versus 3 of 17 female patients (17%; N=3; mean age: 58.6 [6.0] years; mean age at PD onset: 48.3 [2.5] years). A history of major depression or dopamine-induced mood disorder was reported in 4 of 17 patients (23%).
DISCUSSION
Despite the increasing use of DBS as a therapeutic tool for PD, the precise mechanism of action of DBS is currently debated. Since the effects of DBS are similar to those of a lesion, DBS has been thought to silence neurons at the site of stimulation. Reviewed extensively elsewhere,
23,24 hypotheses concerning the mechanism of action of DBS have focused on a state of depolarization blockade at the stimulation site. This DBS-mediated neural network disruption has been hypothesized to result from preferential activation of GABA-ergic inhibitory neurons or high-frequency stimulation-mediated jamming of normal neuronal communication.
25–28 However, there is also evidence to suggest that DBS can elicit neurotransmitter-release distal to the site of stimulation
29–31 and/or effect more enduring plasticity-mediated changes in network function.
32STN DBS Mechanism of Action: Preclinical Models
Bilateral STN DBS typically reduces the need for
L-DOPA treatment and recovers striatal dopamine-receptor function, indicating that dopamine levels have increased.
33,34 This suggests that clinically effective STN DBS mediates dopamine neurotransmission. In support of this idea, striatal dopamine-release during STN DBS has been demonstrated in both intact and dopamine-depleted preclinical animal models.
31,35–39 Furthermore, STN DBS-evoked dopamine efflux has recently been reported in a large-animal (pig) model that faithfully replicates the human DBS neurosurgical procedure and stimulation paradigm.
39 In this study, DBS-mediated striatal dopamine-release was observed to be voltage-dependent, in that, while maintaining constant frequency (120 Hz), increases in the voltage intensity from 3 V to 7 V resulted in a progressive increase in striatal dopamine-release. Animal studies have also suggested that dopamine synthesis may be upregulated by DBS.
37,40Extrapolation of results from preclinical animal models directly to DBS-mediated effects in PD patients, however, must be done with some caution. Differences in the type of electrodes utilized, as well as the scale and anatomy of the rodent brain, somewhat limit translation of the findings. In particular, specific parameters applied to the DBS electrode and the precise location of active leads within the brain can profoundly influence the biological impact of high-frequency stimulation on neuronal activity and neurotransmitter efflux.
41,42Given the potential for dopamine dysregulation to contribute toward behavioral expression of mania in PD,
43,44 observations of up-regulated dopamine receptor function in STN DBS patients and preclinical data demonstrating STN DBS-elicited dopamine-release in motor and limbic basal ganglia circuits,
29,31 the potential for regional STN DBS to contribute to the occurrence of manic symptoms needs to be explored more fully. Toward this end, a recent preclinical primate study has demonstrated that reversible, regional dysfunction of the STN selectively induces movement disorders and radical changes in behavior in healthy animals. In particular, the induction of hyperactivity reliably occurred with inhibition of the anteromedial region of the STN.
45STN DBS Mechanism of Action: Clinical Models
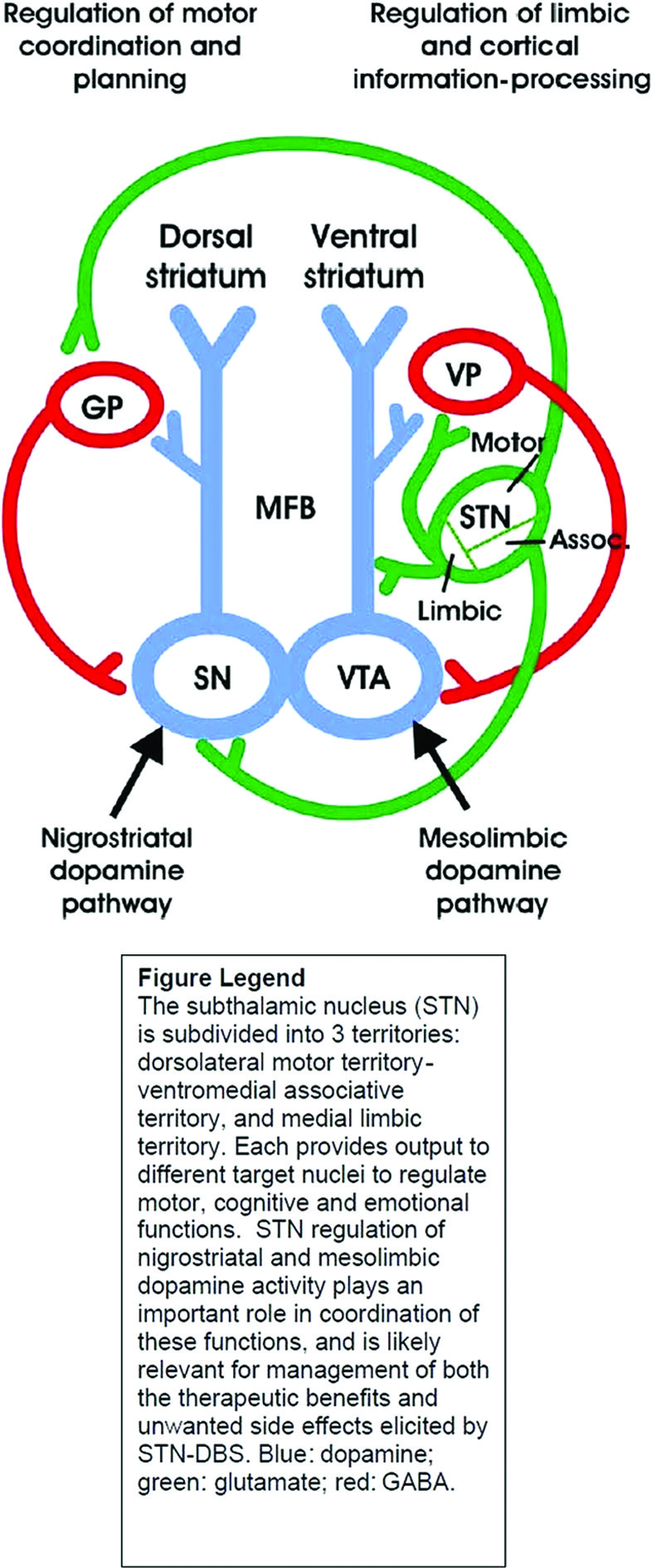
The subthalamic nucleus (STN) is subdivided into three territories: dorsolateral motor territory, ventromedial cognitive territory, and medial limbic territory. Each provides output to different target nuclei to regulate motor, cognitive, and emotional functions. STN regulation of nigrostriatal and mesolimbic dopamine activity helps to coordinate these functions and is likely implicated in both the therapeutic benefits and unwanted PAEs associated with STN DBS. Therapeutic effects of STN DBS in PD are optimized when the electrode is placed in the dorsolateral motor territory. Excitatory glutamatergic efferents project from the dorsolateral motor territory of the STN to the globus pallidus (GP). The GP, in turn, sends an inhibitory GABA-ergic projection to the dopamine cell bodies of the substantia nigra (SN). The SN is also innervated by an excitatory glutamatergic afferent from the ventromedial associative territory of the STN, as depicted in
Figure 1.
46 Thus, DBS activation of these projections could increase dopamine cell activity in the SN and dopamine release in the dorsal striatum to mediate motor function. Conversely, when the DBS electrode is positioned in the limbic territory, excitatory glutamatergic projections to dopaminergic axons of the median forebrain bundle (MFB) may be activated.
15 The axons activated in this instance arise out of dopamine cell bodies in the ventral tegmental area (VTA) and project to the ventral striatum to influence the processing of limbic and cortical information. A glutamatergic projection is also sent from the limbic STN to the ventral pallidum (VP), which in turn sends an inhibitory GABA-ergic projection to the VTA, activation of which would inhibit dopamine cells.
Given that dysregulation of mesolimbic dopamine is implicated in the expression of manic symptomatology, further investigation of the effects of STN DBS on mesolimbic dopamine is much needed, as is determination of its role in STN DBS-induced hypomania.
The role of the STN in regulation of emotional responses and behavior is becoming clearer, and several neuroimaging studies have reported DBS-induced specific changes at the level of cortical and subcortical associative and limbic regions.
47–49 Such a potent influence on associative, limbic, and motor functions has not been observed with other targets, like the GPi
50–53 or the thalamus.
54,55 Also, recent controlled data have suggested that PD patient status post-DBS had differential emotional responses based on dorsal or ventral contact STN stimulation.
56 Emotional reactivity of 20 PD patients given bilateral STN DBS was quantified by assessing emotional reaction to three different movie clips of different valences (neutral, sad, and amusing); ventral stimulation was associated with significantly increased ratings of positive affect, as compared with dorsal stimulation. Not surprisingly, STN stimulation, particularly when contacts are located ventromedially to the intended motor territory, may result in adverse cognitive and neuropsychiatric outcomes, including transient confusion, apathy, reduced verbal fluency, impaired attention, working memory, response inhibition, depression, anxiety, hypomania, hypersexuality, hallucinations, psychosis, and even suicide.
46STN DBS-Induced Mania: Underlying Neurobiology and Clinical Correlates
Application of DBS to the ventromedial STN, particularly with high voltages and/or unipolar stimulation mode, can potentially stimulate adjacent neural structures, including the substantia nigra, ventral tegmental area, and lateral hypothalamus.
14 STN DBS has also been associated with potential stimulation of more formal neural circuits, including orbito-frontal basal ganglia,
12 midbrain-orbitofrontal brain regions, anterior cingulate striato-pallido-thalamo-cortical circuits, or fibers from the ventral tegmentum area to the limbic striatum, causing symptoms typical of mania.
57–59 On the basis of evidence from diffusion tensor imaging (DTI), STN DBS-induced reversible acute hypomania might be elicited by inadvertent and unilateral coactivation of putative limbic STN tributaries to the medial forebrain bundle (MFB), a recently-identified pathway connecting the medial STN to reward circuitry.
13A recent study, focusing on contact-dependent reproducible hypomania in five PD patients, demonstrates the role of subcortical structures, specifically the substantia nigra, in the genesis of STN DBS-induced hypomania. Three-dimensional anatomical reconstruction with multimodal imaging (preoperative stereotactic MRI, postoperative CT scan, oxygen-15 PET scan) of “euthymic” and “manic” contacts revealed that 9 of 10 “manic” contacts were located in ventral substantia nigra, with right anterior cingulate and medial prefrontal cortex activation.
17The observation of bilateral globus pallidus DBS-induced mania in a patient with no previous psychiatric history
60 suggests that there may be other stimulation sites outside of STN that have potential to cause this psychiatric adverse event. Because of the much-less-reported burden of PAEs associated with GPi stimulation; it may be an intuitive target choice for DBS stimulation in patients at risk of developing DBS-induced PAEs. However, these data are limited, and a higher rate of suicide in a 10-year meta-analysis has been reported with GPi stimulation.
61The literature reviewed to-date, would suggest that STN DBS-induced hypomania/mania seems to be most consistently associated with ventromedial STN electrode placement. The reproducibility of mania with ventral stimulation, resolution of manic symptoms with dorsal stimulation in 86% of cases (a change that also shifts the stimulated site laterally, given the typical DBS lead trajectory), and persisting manic symptoms despite significant reduction in LEDD further confirm this association. In terms of stimulation parameters, higher stimulation voltage (>3 V) and/or unipolar stimulation mode have been associated with STN DBS-induced mania. The voltage-dependent nature of STN DBS-induced mania
10 replicates recently-reported voltage-dependent dopamine efflux in a large-animal model of DBS.
39 Men and/or those with early-onset PD may be at significant risk of developing treatment-emergent adverse events to a number of PD treatment interventions. In a multicenter study on suicide outcomes after STN DBS for Parkinson's disease;
62 younger age and early-onset PD were significantly associated with STN DBS treatment-emergent suicide attempts. Furthermore, in a cross-sectional evaluation of patients evaluated in a Movement Disorders Clinic, men were more likely to develop dopamine agonist-induced impulse-control disorders.
63 These findings may suggest that men and/or early-onset PD may represent the PD population at the most risk of developing dopamine-agonist (whether medication- or DBS-induced) PAEs such as mania and suicidality.
Somewhat surprising, a past psychiatric history of depression or dopamine-induced mood disorder was only present in 4 of 17 reported cases (23%). It may be possible that STN DBS may have unmasked symptoms of preexisting psychiatric illness that were not identified in baseline assessment.
64 Patients with preoperative cognitive deficits and affective disorders seem to be at risk of behavioral problems after STN DBS surgery.
65,66 A recent small study of 14 PD patients undergoing STN DBS suggested that baseline motor impairment was associated with post-surgical hypomania symptoms,
67 as it has already been suggested that sudden improvement in motor function after DBS surgery may have a profound effect on mood.
68Limitations
We acknowledge that psychiatric side effects after STN DBS surgery are published mostly in case-report format; therefore, their frequency might be overestimated.
22,69,70 But it might equally well be the case that frequency of such side effects is underestimated, as these side effects are not subjected to systematic assessment. The precise mechanism(s) of action of STN DBS-induced hypomania/mania are currently not well understood. Our findings/proposed mechanisms may still be preliminary and speculative, and this needs further systematic research.
CONCLUSION
STN DBS-induced mania and other adverse events emphasize the need for comprehensive presurgery psychiatric evaluation and close follow-up after implantation. Given the significant psychiatric morbidity and mortality associated with STN DBS surgery in PD patients, there is need for preclinical animal studies and prospective cohort/ longitudinal studies, combined with advanced neuroimaging techniques, to specifically establish psychiatric, neurological, and neurosurgical correlates and elucidate the neurobiological mechanism(s) associated with STN DBS-induced mania. Such working models would serve to further our understanding of the neurobiological underpinnings of mania and contribute valuable new insight toward development of future DBS mood-stabilization therapies.
Acknowledgments
This work was presented in part at the American Neuropsychiatric Association Annual Meeting, Tampa, FL, March 18, 2010.
We thank Ms. Cindy Stoppel for her help in the preparation of this manuscript.
Dr. Tye was supported by a NARSAD Young Investigator Award.
Mark A. Frye, M.D. has grant support from Pfizer, National Alliance for Schizophrenia and Depression (NARSAD), NIMH, National Institute of Alcohol Abuse and Alcoholism (NIAAA), and the Mayo Foundation, and is a consultant to Dainippon Sumittomo Pharma, Merck, and Sepracor. CME-supported activity: Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Merck, Otsuka Pharmaceuticals, Pfizer, and Sanofi-Aventis.
Dr. Kendall H. Lee has patents pending relating to DBS technology and has industry support from St. Jude Neuromodulation Division. This work was supported by NIH (K08 NS 52232 award) and Mayo Foundation (2008-2010 Research Early-Career Development Award for Clinician-Scientists) to KHL.
Julie A. Fields, Ph.D., is a consultant to Medtronic, Inc.