Methamphetamine (MA) is a potent, highly addictive, and neurotoxic psychostimulant with well-documented adverse neural and neuropsychological effects,
1 as well as risk of functional declines.
2 Relative to other neurotoxic substances, MA may be particularly potent because of its high lipid-solubility and rapid diffusion across the blood–brain barrier.
3 As a result, MA-associated central nervous system (CNS) effects can include a variety of metabolic, structural, and functional brain changes that may have an effect on cognitive functioning. Although some evidence suggests that partial neural and cognitive recovery may occur with stable abstinence,
4,5 alterations and impairments may nevertheless persist long after MA use is discontinued.
6,7 Long-term effects of MA may be especially problematic in frontostriatal circuits, where potential consequences include neural injury in the striatum
4 and prefrontal cortex.
8 It has been hypothesized that these neural changes may affect the modulatory functions of the frontostriatal and limbic structures, as well as underlie the neurocognitive deficits observed in chronic MA users.
9Approximately 40% of chronic MA users exhibit neurocognitive deficits,
10 with generally moderate impairments observed in the domains of episodic memory, motor skills, language, information-processing speed, visuospatial functioning, and executive functions.
1 Within the domain of executive functions, MA has been associated with impulsivity, disinhibition, reduced ability to suppress irrelevant information, risky decision-making, and increased distractibility.
7,11,12 Neurocognitive impairments, and particularly executive dysfunction, may increase the risk of everyday functioning problems and engagement in high-risk behaviors, which are common among MA users. Self-report measures indicate lower abilities and more disruptions in everyday activities including communication, work, and recreation.
13 These data are corroborated by significant impairments found on performance-based tests, including tests of medication and financial management, arranging travel, and communication skills.
2 MA use has also been associated with the lack of health insurance, being on public assistance, and disproportionate representation in burn and trauma units.
14 MA use is negatively correlated with employment stability, and MA users are less likely to be employed full-time.
15 Chronic MA users report elevated rates of psychosocial problems, including domestic conflict
16 and legal complications.
17 Of course, there are long-standing controversies regarding the construct validity of such self-reported versus more objective (i.e., performance-based or observational) assessments of everyday living problems such as these.
18 Although self-report approaches are limited by potential bias and current mood, they may also be more sensitive to gross functional difficulties. Also, self-report measures may more flexibly identify declines specific to the demands of each individual's life.
19 As such, assessing self-reported neurobehavioral symptoms may nonetheless yield clinically meaningful and incrementally important information with limited investment of time and minimal examiner burden.
To our knowledge, the prevalence and correlates of neurobehavioral disturbances in chronic MA users have not yet been fully established. Here, we define neurobehavioral disturbances as self-reported behavioral symptoms that have reliably been associated with brain injury and neuropsychiatric syndromes, particularly those involving dysregulation of frontostriatal systems.
20 Clinicians frequently note behavioral disturbances in MA, and broadly accept that this impulsivity, disinhibition, and predisposition toward stimulus-driven decision-making may reflect the observed neuropsychological deficits and frontal-systems injury sustained by MA users. Importantly, studies have found these types of behaviors to be independently related to adverse psychosocial, psychological, substance-related, and sexual risk factors. For example, higher levels of impulsivity in MA users have been associated with elevated rates of psychiatric diagnoses, higher rates of unemployment, high-risk sexual encounters, and a greater tendency to use MA to cope with mood disturbances.
21,22 Despite the suggestion that these neurobehavioral symptoms are uniquely associated with important outcomes, the nature and magnitude of neurobehavioral disturbances and their effects on everyday functioning have not been extensively studied in this population.
Although some existing studies
23–25 have investigated neurobehavioral symptoms in substance-using populations, these designs have included heterogeneous groups of polysubstance abusers, rather than examining substance-specific risks (e.g., MA dependence). Given the severity of the aforementioned neural, cognitive, and functional effects in MA relative to other substances, the primary aim of the current study is to examine, by use of the Frontal Systems Behavior Scale (FrSBe),
20 the presence and severity of neurobehavioral symptoms (e.g., apathy, disinhibition, and executive dysfunction) in individuals with a history of MA dependence. The FrSBe has been used to quantify significant neurobehavioral sequelae in other conditions affecting similar brain regions and neural pathways (e.g., Parkinson's disease
26). We also sought to explore the potential risks that may be associated with elevated neurobehavioral symptoms in MA, including neurocognitive and functional impairment. Neurobehavioral symptoms themselves are an important indicator of an individual's functioning, and, as in other populations (e.g., dementia patients)
27 could predict everyday functioning outcomes over and above any observed cognitive deficits. By clarifying the nature and severity of behavioral disturbances in MA users, clinicians may be able to better identify those at risk for functional impairments and better anticipate and account for difficulties in complying with intervention programs.
Method
Participants
A total of 158 eligible participants were included in this study. Participants in the MA+ group met lifetime diagnostic criteria for MA dependence via semistructured diagnostic interview (Composite International Diagnostic Interview; CIDI Version 2.1)
28 and met criteria for MA abuse within the last 18 months (N=73). Participants in the MA− comparison group (N=85) had never met criteria for MA-dependence and reported no previous use of MA, but had been recruited to match the MA+ group on demographic and other substance-related risk factors (e.g., depression) wherever possible. A minimum of 10 days of abstinence from MA was required before testing, and a urine toxicology screen confirmed that participants had abstained from use of all illicit substances except marijuana. Marijuana is detectable for up to 30 days after last use, and because of the high comorbidity of marijuana abuse and dependence in MA users, was not considered exclusionary for study participation in either group. Substance-related exclusion criteria included meeting
Diagnostic and Statistical Manual (DSM-IV)
29 criteria for current (within 30 days) abuse or dependence of non-MA substances. Individuals with histories of alcohol dependence within 1 year of evaluation, or other substance use/dependence within 5 years of evaluation, were also excluded. Potential participants were excluded if they reported a past head injury with a loss of consciousness greater than 30 minutes, HIV infection, a history of neurological condition (e.g., seizure disorder, stroke) or psychiatric illness (e.g., mental retardation or schizophrenia-spectrum diagnoses) affecting cognitive functioning. All participants in the current study were HIV-seronegative, as determined by enzyme-linked immunosorbent assay (ELISA). Given the prevalence of hepatitis C virus (HCV) in substance-abusing populations, HCV-infected individuals were included in the analysis. HCV serostatus was determined by standard clinical antibody detection, and HCV RNA was measured in serum using real-time polymerase chain reaction (NGI SuperQuant; National Genetics Institute, Los Angeles, CA, USA; nominal detection limit of 100 IU/mL).
The MA+ and MA− groups were comparable on demographic factors (p values >0.10; see
Table 1). They did not significantly differ on HCV serostatus or lifetime rates of major depressive disorder. As might be expected, the MA+ group had higher lifetime rates of alcohol, cannabis, and cocaine dependence (all p values <0.05), but did not significantly differ on histories of any other substances. Although the MA+ group had a significantly higher proportion of individuals diagnosed with ADHD (p <0.05), differences between the proportions of individuals meeting criteria for antisocial personality disorder (ASPD) approached significance (p<0.07).
Materials and Procedure
Psychiatric and Substance Abuse Factors
Table 1 presents the psychiatric and substance-use characteristics of the two study groups. MA use characteristics (duration and recency) were obtained via a semistructured timeline follow-back interview.
10 Trained interviewers administered the CIDI,
28 which is a semi structured, computer-assisted interview that provides lifetime and current substance use and psychiatric (e.g., major depressive disorder) diagnoses according to DSM-IV criteria. ASPD was diagnosed with the SCID,
30 and ADHD diagnoses were determined using the Diagnostic Interview Schedule.
Neurobehavioral Symptoms
As part of their evaluation, all participants completed the self-report version of the Frontal Systems Behavior Scale (FrSBe),
20 a 46-item, self- or other-report behavior rating scale that provides quantitative measurement of behavioral disturbances related to damaged frontal systems. The FrSBe yields a total score, in addition to three subscale scores: apathy, disinhibition, and executive dysfunction. Behaviors for apathy (e.g., “sit around doing nothing;” “show little emotion, am unconcerned and unresponsive”), dysregulation (e.g., “laugh or cry too easily;” “talk out of turn; interrupt others in conversation”), and executive dysfunction (e.g., cannot do two things at once [for example, talk and prepare a meal];” “show poor judgment; poor problem-solver”) are rated on a 5-point Likert-type scale from 1 (“almost never”) to 5 (“almost always”), where higher scores correspond to more abnormal behavior. Because of the inclusion of retrospective as well as current ratings, the FrSBe also enables the comparison of pre- and post-injury responses. This feature is frequently utilized in patients with acute symptom-onset (e.g., after head injury or a surgical intervention), but the current study included only current ratings because of the diffuse nature and insidious onset of MA-related pathology. This practice is consistent with a number of other investigations in which the FrSBe was used to assess behavioral symptoms of neurological conditions that present more gradually (e.g., HCV).
31 Total and subscale raw scores were converted into demographically-adjusted T scores for analysis. As indicated by the measure’s authors, a T score cut-point of 65-or-higher was used as an indicator of clinically elevated behavioral symptoms. FrSBe T scores for the Total score and three subscales (Apathy, Disinhibition, Executive Dysfunction) were the primary dependent variables of interest.
Dependence on Activities of Daily Living
Participants also completed a modified version of the Lawton and Brody Activities of Daily Living Scale.
32 This instrument is designed to assess participants’ current functioning and identify improvements or declines relative to their best-ever level of functioning in eight areas related to routine daily tasks (e.g., employment, financial management). Participants rated each item on a 3- (0–2) or 4- (0–3) point scale, with higher scores indicating poorer functioning. For the current study, a total score was generated to represent the total severity of declines reported in current versus past functioning on all tasks assessed by the measure (range: 0–11).
Neurocognitive and Neuromedical Assessments
All participants provided informed consent before completing comprehensive neurocognitive and neuromedical assessments. The neuropsychological assessment covered seven cognitive domains: verbal fluency, working memory, speed of information-processing, learning, recall, executive functions, and fine motor coordination. Iudicello et al.
5 provide a complete list of neuropsychological tasks included in each domain. Raw test scores were then converted into demographically- (age and education) corrected T scores before deficit scores were computed for each domain. Individual domain deficit scores were averaged to obtain a global deficit score (GDS),
33 in which higher scores reflect poorer neurocognitive performance.
Data Analysis
Because of the non-normality of the variables of interest (FrSBe T scores; Shapiro-Wilk test; p values <0.01), all primary between-group analyses were conducted using nonparametric tests. First, a series of Wilcoxon ranked-sum tests and Cohen’s
d statistics
34 were used to compare behavioral symptoms (i.e., FrSBe Total and Apathy, Disinhibition, and Executive Dysfunction T scores) in MA+ and MA− groups. Chi-square tests and odds ratios (ORs) were calculated to examine the effects of MA dependence on clinically elevated FrSBe scales (T scores >64).
20 Next, a planned series of regression analyses were conducted to examine the unique effects of MA group on FrSBe variables, accounting for the effects of potentially confounding substance-use factors on which the groups differed (i.e., lifetime diagnoses of cocaine, cannabis, and alcohol dependence). Because of the limited prevalence of HCV, ADHD, and ASPD in the MA− group, associations between these conditions and the FrSBe ratings were conducted only within the MA+ group, using Wilcoxon ranked-sum tests. Nevertheless, it is important to note that the effect of MA+ group status on FrSBe T scores remained significant in a logistic regression in which these factors were included. Spearman’s rho (ρ) correlational analyses within the MA+ group were used to explore potential associations between FrSBe ratings and cognitive variables (GDS and domain deficit scores) and MA-use characteristics (i.e., last use, cumulative duration and quantity of use, age at first use, and age at onset and recency of MA-dependence diagnosis). Finally, a series of multiple-regression analyses were used to examine each of the FrSBe variables as independent predictors of IADL dependence within the MA+ group while accounting for standard cognitive (GDS), medical (e.g., HCV status), psychiatric (e.g., current major depressive disorder), and MA-use characteristics (i.e., last use of MA) known to be associated with IADL decline. The critical alpha level was set to 0.05 for all analyses.
Results
Table 2 presents the means, standard deviations (SDs), and Cohen’s
d effect sizes for the FrSBe variables. Relative to the non–MA-using comparison participants, the MA-dependent group endorsed a significantly greater level of overall behavioral disturbance (FrSBe Total T score p<0.002;
d=0.51), Disinhibition (p<0.001;
d=0.64) and Executive Dysfunction (p<0.001;
d=0.61), but not Apathy (p>0.10). As shown in
Table 3, MA group remained a significant predictor of the Total FrSBe T score, as well as the Disinhibition and Executive Dysfunction subscales (p values <0.01) even when accounting for potentially confounding factors on which the groups differed. Although the proportion of individuals with comorbid ADHD diagnoses was significantly higher in the MA+ group than in the MA− group, total and subscale T scores and proportions of clinically elevated scores were comparable between MA+ individuals with and without comorbid ADHD. There were no significant effects of ASPD on any FrSBe scale within the MA+ group. Finally, there were no significant differences between total and subscale T scores or proportion of clinically elevated individuals between MA+ individuals who were and were not infected with HCV.
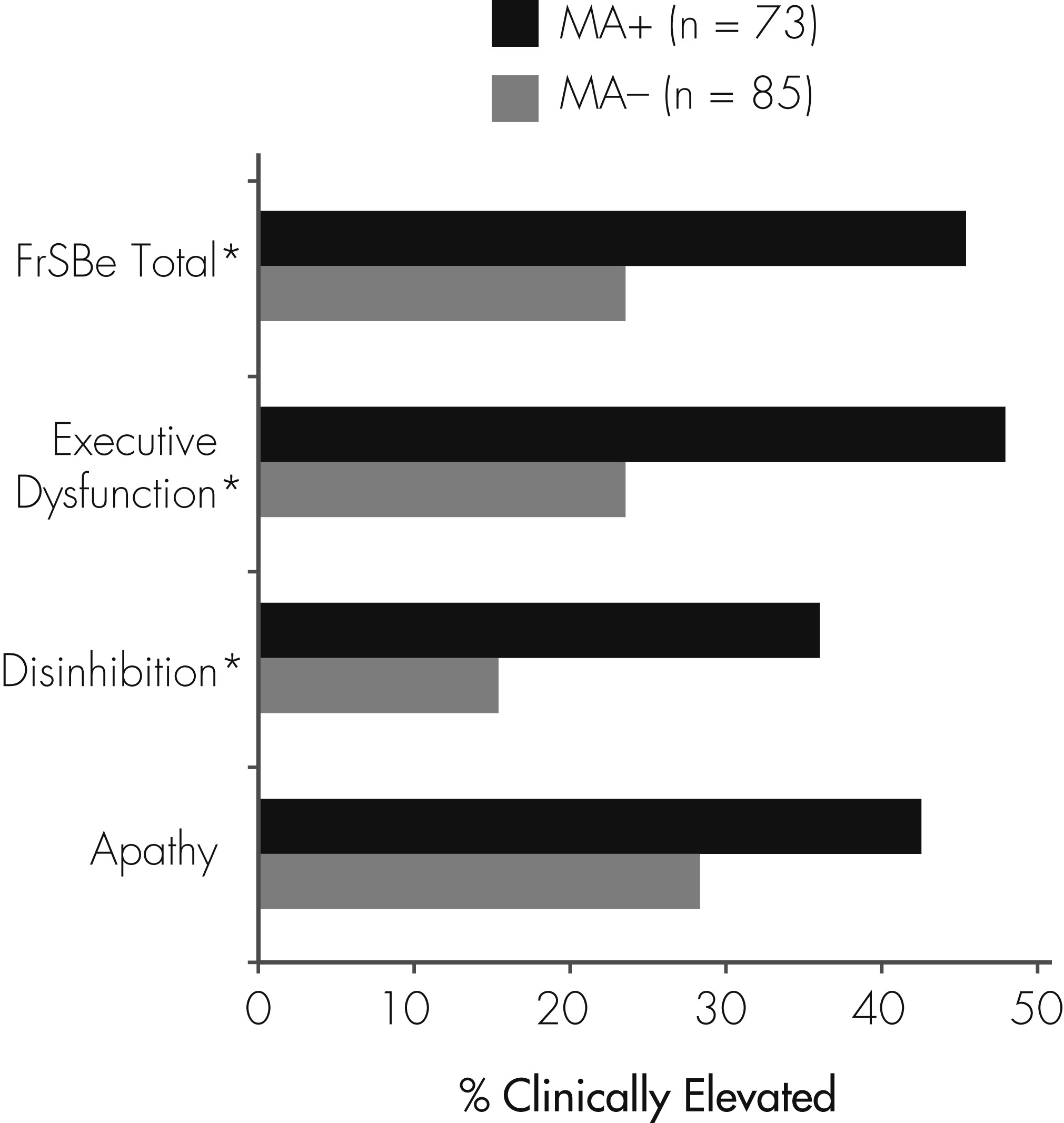
At the group level, the MA+ group had a significantly higher proportion of individuals with clinically elevated T scores on FrSBe Total (45.2% versus 23.5%), Disinhibition (36.1% versus 15.3%), and Executive Dysfunction subscales (48.0% versus 23.5%) relative to the non-MA users (all p values <0.01; see
Figure 1). ORs revealed that a history of MA dependence was associated with an approximately threefold risk of clinical elevations on the FrSBe. ORs were 2.68 for Total FrSBe (95% confidence interval [CI]: 1.37–5.37), 3.13 for Disinhibition (95% CI: 1.48–6.87), and 2.99 (95% CI: 1.53–5.99) for Executive Dysfunction.
Within the MA+ group, no significant correlations were found between FrSBe T scores and GDS or other domain deficit scores. No significant correlations were observed between FrSBe T scores and MA-use parameters (e.g., age at first use, cumulative quantity, cumulative days in which methamphetamine was used) in the MA+ group.
Finally, MA-associated behavioral symptoms were predictive of functional decline even while accounting for standard cognitive, medical, psychiatric, and MA-use variables that have previously been associated with IADL decline. Specifically, within the MA+ group, identical linear-regression models identified the FrSBe Total T score (β=0.37; p
<0.002), Apathy subscale T score (β=0.29; p <0.005), Disinhibition subscale T score (β=0.22; p <0.04), and Executive Dysfunction subscale T score (β=0.36; p<0.0003) as significant, independent predictors of IADL decline severity (see
Table 3).
Discussion
Chronic MA use has been associated with neurocognitive and psychosocial complications and adverse neurobehavioral symptoms,
7,12 potentially reflecting MA-associated neural damage to frontostriatal systems. Moreover, these behavioral symptoms have been linked to MA-specific risk behaviors
21 and adverse psychosocial outcomes.
22 This study extends the literature by demonstrating an increased prevalence and greater severity of self-reported behavioral symptoms in MA-dependent individuals relative to non-MA users. Specifically, we found medium-to-large differences between MA-dependent individuals and their non–MA-using counterparts in terms of overall self-reported neurobehavioral symptoms as well as elevated ratings of self-reported disinhibition and executive dysfunction. In fact, the MA-dependent individuals as a group had a significantly greater proportion of individuals reporting clinically elevated T scores overall, relative to the non-MA users; that is, MA users were approximately three times more likely than the comparison subjects to report a clinically elevated level of disinhibition and executive dyscontrol. This is consistent with evidence of the behavioral disturbances (e.g., impulsivity and discounting of monetary rewards) observed in other stimulant-using populations
35 and the higher levels of neurobehavioral symptoms reported on the FrSBe by polysubstance-using individuals.
36To our knowledge, whereas research has identified neurobehavioral disturbances that are associated with substance dependence,
23 this was the first study to examine potential MA-specific neurobehavioral symptoms assessed with a well-validated, easily administered clinical questionnaire and the first to examine these neurobehavioral symptoms as predictors of declines in everyday functioning. Our results speak to the unique risk posed by MA, even in the context of other substances and comorbidities, including psychiatric disorders (e.g., ADHD, ASPD, and MDD) and infectious disease (e.g., HCV). Given the high rates of comorbid substance abuse and dependence in MA-using populations, we did not exclude MA-users who also reported current and past use of other substances. To control for these effects, we utilized a comparison sample of individuals who were broadly comparable in terms of substance histories, rather than healthy individuals. Moreover, the effect of the MA group remained even when dependence on other substances of abuse was included in the statistical models. Thus, the robust effect sizes that we observed between MA+ and MA− groups provide evidence for an independent effect of MA on neurobehavioral symptoms that may be separable from the effects of other illicit-substance use.
No significant differences in apathy were observed between the MA+ and MA− individuals. This finding was unexpected, given clinically elevated means for apathy symptoms in clinical samples with high rates of stimulant use, including those with HIV
37 and HCV.
38 However, other investigations of polysubstance-dependent individuals have also found trend-level effects for apathy symptoms similar to what we observed.
23 One possible reason for the lack of significant findings on the Apathy subscale is the common utilization of amphetamines to treat apathy in a number of disorders (e.g., multiple sclerosis).
38 Thus, apathy symptoms may not be as pronounced in MA users. Future work is nevertheless necessary to further investigate whether risk for apathy symptoms increases with prolonged abstinence from MA use.
Also contrary to our expectations, we found no significant correlations between neurocognitive deficits and neurobehavioral symptoms in MA+ individuals. However, the observed relationship between neurobehavioral symptoms and everyday functioning in MA users suggests that, as in other conditions (e.g., dementia),
27 neurobehavioral symptoms may provide unique information about an individual’s functioning beyond that which can be gleaned from their neuropsychological profiles. In fact, there is evidence from lesion studies to suggest that the expression of cognitive deficits and clinically observable neurobehavioral symptoms is dissociable at the neural level.
39 Furthermore, correspondence between performance-based and self-report measures is poor in clinical populations.
40 Future studies may continue to explicate this apparent “disconnect” between neuropsychological performance and neurobehavioral symptoms.
Of particular clinical relevance, MA-associated neurobehavioral symptoms were uniquely associated with declines in instrumental activities of daily living. Specifically, higher levels of overall neurobehavioral disturbance, as well as elevated ratings of disinhibition and executive dyscontrol, were each independently predictive of more severe IADL declines in the MA+ group. This is consistent with research demonstrating both behavioral disturbances
22 and compromised everyday functioning
13 in MA-dependent individuals. These effects were noted independently of well-established predictors of everyday functioning, indicating the potential importance of neurobehavioral symptoms in predicting the IADL declines that may occur in MA+ individuals.
2 Results of this investigation suggest that behavioral disturbances in MA users, although often noted anecdotally in clinical settings, may serve as important indicators of IADL difficulties. These data speak to the potential adjunctive ecological value of self-reported behavioral disturbances when given alongside a standardized neuropsychological and psychiatric assessment of clinical symptoms. In particular, well-validated instruments such as the FrSBe may be useful in this regard. By measuring these neurobehavioral symptoms in MA+ individuals, clinicians and researchers may be able to identify those at risk for poorer functioning and diminished capacity to operate independently in important activities of daily living (e.g., managing finances, cooking, medication management). Future work may extend the current literature by exploring whether other objective everyday functioning outcomes (e.g., credit card debt, employment) are associated with neurobehavioral symptoms in MA+ individuals. Further exploring the relationship between neurobehavioral symptoms and everyday functioning in chronic MA users may allow providers of rehabilitation services to better identify and target behavioral problems that may subsequently impair aspects of social and occupational functioning.
Although the current study provides strong evidence for sizable neurobehavioral disturbances associated with MA dependence, important etiological questions remain and serve as potential targets for future work. For instance, it is not yet clear whether elevated neurobehavioral symptoms are directly indicative of MA-related toxicity to frontal systems, although some functional neuroimaging studies demonstrate behavioral differences associated with prefrontal dysfunction in MA-dependent individuals.
41 It is known that these neurobehavioral symptoms are not specific to frontostriatal circuit involvement, as they have been observed in multiple sclerosis and other disorders with vastly different effects on frontal systems.
42 For this reason, future investigations may incorporate functional and structural neuroimaging methodologies in order to link frontal-systems compromise more directly to neurobehavioral symptom elevations in MA users.
A few limitations of the current study can also be addressed in future work. For instance, although the high proportion of HCV-infection in our sample is representative of community samples, HCV has been associated with elevated neurobehavioral symptoms in non–stimulant-using samples.
31 Although we did not observe an HCV effect in our MA sample, prospective studies controlling for important co-factors (e.g., demographics) are needed to more carefully determine the potential additive or synergistic effects of MA and comorbid infectious disease, perhaps also including HIV infection.
10 Also, the current protocol did not collect the caregiver or clinician reports on the FrSBe. Other investigations might prioritize the collection of these data in order to compare self-reported neurobehavioral symptoms with those reported by others. However, as caregivers and clinicians tend to report higher levels of behavioral symptoms than do substance users,
20 we would expect any differences to occur in the conservative direction. Also, the current study design did not include an analysis of participants’ self-reported neurobehavioral symptoms before their MA use because of their early age at first use and insidious onset of MA-related changes. Given research to suggest that higher levels of trait impulsivity may serve as both a facilitator and a consequence of substance abuse,
43 future research is necessary in order to investigate whether individuals with premorbid neurobehavioral symptoms are more likely to have elevated symptoms during MA dependence and after long-term abstinence.