Converging lines of evidence suggest that the rostral anterior cingulate cortex (rACC) plays a crucial role in the neurobiology of major depressive disorder (MDD) and that activity in this region predicts response to antidepressant treatment. Patients suffering from MDD who respond to medication show elevated pretreatment activity in the rACC relative to non-responders.
1–6 The relationship between rACC activity and treatment response has been demonstrated by multiple imaging modalities, including positron emission tomography (PET),
1,3 and with quantitative electroencephalographic (qEEG) source localization, using low-resolution brain electromagnetic tomography (LORETA).
2,4,5,7Elevated resting state rACC activity has been hypothesized to reflect a greater ability to engage in adaptive self-referential processing, thereby conferring better prognosis for antidepressant treatment outcome.
6 It is unknown whether this neurophysiologic marker remains fixed, or whether resting state rACC activity may change over the course of a single depressive episode, thus providing a variable or “state” marker of responsiveness. This question is of interest because, to the extent that rACC activity may constitute a state marker, there may be critical times in the course of illness in which treatment would be more likely to be effective. It also might be possible to alter rACC activity via the manipulation of state, for example, through cognitive interventions, mindfulness practices, or other methods of self-regulation, in order to enhance response to treatment.
Previous reports have not examined rACC activity on more than one occasion before treatment. Therefore, it is not known whether the relationship between rACC activity level and clinical response depends on the time of the rACC assessment. If rACC activity varies over time and reflects a state marker of responsiveness, then we would expect that the relationship between rACC activity and antidepressant response depends on the timing of the rACC measurement. Measurements closer in time to the beginning of antidepressant treatment would better reflect the “state” of the individual at treatment initiation, and therefore would be expected to be better predictors of outcome of a course of antidepressant treatment.
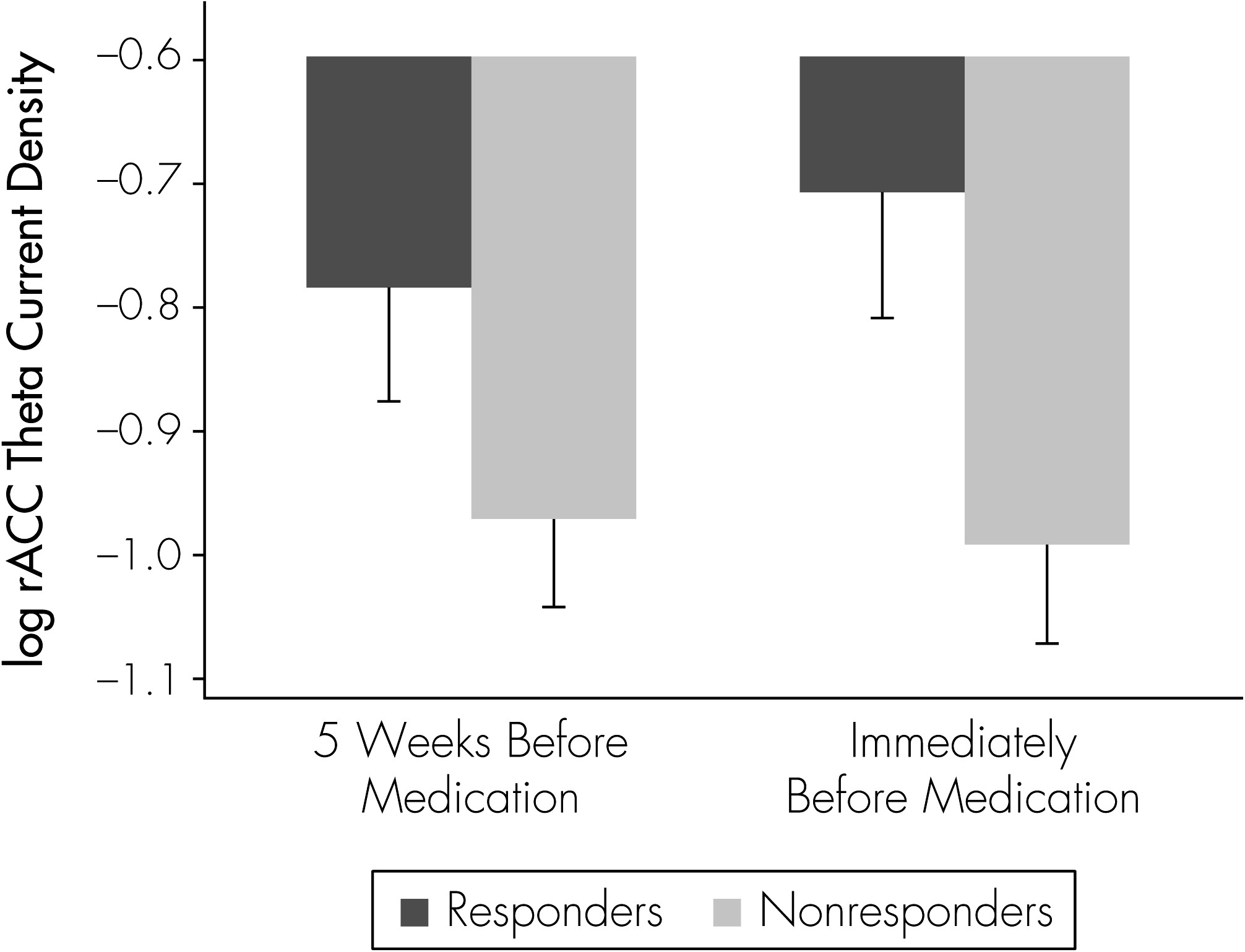
The current study examined rACC activity at two time-points: 5 weeks before the beginning of antidepressant treatment, and again immediately preceding the start of treatment. We hypothesized that if rACC represents a state marker of responsiveness, then the relationship between antidepressant response and rACC activity would depend on the timing of the rACC measurement.
Methods
Study Design
We examined brain electrical activity in 22 subjects who had failed to respond to 5 weeks of double-blinded placebo treatment and who then were crossed over to blinded treatment with antidepressant medication. EEG measurements were taken before the placebo treatment phase (5 weeks before crossover to medication) and again immediately before medication treatment was initiated. This study design allowed us to examine rACC brain functioning measured on two separate occasions before the start of antidepressant treatment, under conditions where neither EEG was confounded by psychotropic medications.
All subjects had been enrolled in a two-phase, placebo-controlled treatment trial of MDD (ClinicalTrials.gov #NCT00229476) primarily designed to provide insights into the placebo effect in MDD. The first phase of the trial consisted of an initial week of single-blinded placebo treatment, followed by 5 weeks of double-blinded treatment with placebo or medication (sertraline), with 3:1 randomization favoring placebo. In the second phase of the study, placebo non-responders from the first phase of the study (17-item Hamilton Rating Scale for Depression [Ham-D–17] >10) underwent a blinded crossover to medication.
Double-blind treatment was maintained throughout the study. Subjects were told that they would receive placebo or medication at the outset of the study, that all subjects would be on placebo at some point, and, that changes to their medication might be made at the study midpoint. Neither the subjects nor the study personnel who had contact with them were informed of the study design, nor who was receiving placebo or medication either before or after the crossover. Blinded crossover was facilitated by providing the research pharmacy with each subject's Ham-D–17 score after the first phase of the study. Study material (i.e., capsule containing either sertraline or placebo) was supplied by the research pharmacy on the basis of this score. Subjects in the parent study who responded to placebo in the first phase remained on placebo during the second phase. Those who did not respond to placebo in the 5 weeks of the study were switched to sertraline; these subjects are the focus of our present report.
Sertraline was titrated from a starting dose of 50 mg (1 capsule), with a 50 mg increase on Day 8 (2 capsules), and a final 50 mg increase on Day 15 (3 capsules), to achieve 150 mg. Blinding was preserved by matching the number of capsules taken in the placebo and sertraline conditions. At the point of crossover, each subject, regardless of whether she or he remained on placebo or was crossed over to sertraline, was provided with two pill bottles and was given instructions to cross-titrate from the first pill bottle to the second. Each subject was informed of his or her treatment assignment at the end of the second phase (Week 10) by a staff member not otherwise associated with the study.
In the first phase of the study, 69 subjects had been randomized to placebo, and 45 completed the first 5 weeks of treatment; 30 of these subjects did not respond and therefore were crossed over to sertraline in the second phase of the study; these 30 subjects represented the potential pool for the present report. Of the 30 subjects, 6 were missing at least one EEG, and 2 did not complete medication treatment and therefore were excluded from this analysis. For the 22 remaining subjects, data were analyzed from both EEG recordings, one of which was performed 5 weeks before the other, immediately before the start of medication.
Subjects
Subjects were recruited from outpatient clinics of the UCLA Neuropsychiatric Hospital and through community advertisements. At entry, all subjects had Ham-D–17 scores
>16
8 and met DSM-IV criteria for MDD, diagnosed with the Mini-International Neuropsychiatric Interview for DSM-IV.
9 All subjects were between 21 and 65 years of age and in good physical health (i.e., free of any medical condition known to affect brain function). Demographics of the sample are shown in
Table 1. Subjects were excluded if they received any medication known to significantly affect brain function, if they had a history of suicide attempt, or if they had previously failed to benefit from treatment with sertraline. Subjects were free of any psychotropic medication for at least 2 weeks before enrollment. All experimental procedures were approved by the UCLA Institutional Review Board, and each subject provided written informed consent after receiving a full explanation of the procedures.
Clinical Assessment
Clinical symptoms were assessed with the Ham-D–17 at weekly visits. Subjects were classified as medication responders if they showed a ≥50% reduction in Ham-D–17 score over the course of 5 weeks of treatment with sertraline.
EEG Procedures
EEG recording methods have been described previously.
5,10,11 We utilized an extended International 10–20 System with an electrode cap of 36 recording electrodes (ElectroCap, Inc.; Eaton, OH) referenced to Pz. Electrode impedances were balanced and under 5kΩ for all channels. The Neuroscan System (Compumedics, Inc.; El Paso, TX) was used to collect up to 20 minutes of EEG data while subjects rested in the eyes-closed, maximally-alert state in a sound-attenuated room with subdued lighting. Patients were frequently and vigorously realerted at the first sign of drowsiness. EEG data were digitized on line at 250 samples/channel/sec., with a high-frequency filter of 70 Hz and a low-frequency filter of 0.3 Hz, as well as a notch filter at 60 Hz. Only EEG that was free of eye movement, drowsiness, excess muscle, or other artifacts was considered for further processing; an EEG technologist selected for processing the first 10 to 30 2-second epochs of data that met this criteria. Twenty seconds of EEG has been shown to be sufficient for characterizing clinically-relevant differences in depression.
10,12–14 Segments of the recording likely to represent artifact were identified, based upon voltage step gradient, absolute values of difference within the epoch, or persistent low activity. A semi-automated interactive process was used to remove all epochs containing eye movement-, muscle-, or movement-related artifacts, or amplifier drift. Before further processing, a second EEG technologist, blinded to subject identity and treatment condition, independently confirmed that only artifact-free data were selected. EEGs for which 20 seconds of artifact-free data could not be obtained were discarded from further processing. The average number of epochs selected per EEG recording was 17.6 (standard deviation [SD]: 5.5).
LORETA Analysis
The LORETA-KEY package (
http://www.unizh.ch/keyinst/loreta)
15 was used to compute theta-band (4–7 Hz) current density for each subject. Our LORETA methods have been described previously
5 and are summarized here. A cross-spectral matrix was used to compute current density in each of 2,394 cortical voxels. For each subject, theta-band current density was normalized to a total power of 1.0.
Based on previous studies in our lab and others, a region of interest (ROI) was created for the rACC (
Figure 1). The rACC ROI consisted of 14 voxels that had previously been shown to differentiate medication responders from non-responders.
2,5 All 14 voxels were averaged together to create a single current density value, which was log-transformed to more closely approximate a normal distribution.
Statistical Analysis
Analyses were carried out using Stata 12 (StataCorp LP; College Station, TX). To examine the differences in rACC theta-current density between medication responders and non-responders, we conducted multilevel mixed-effects regression analysis, with rACC theta-current density as the dependent variable. The regression included the factors of Time (2 levels: immediately before or 5 weeks before medication treatment) and Response (2 levels: responders or non-responders), as well as their interaction, all modeled as fixed effects. Because age and treatment history were found to be associated with response in this cohort (see Subject Demographics section), and because antidepressant treatment history has been shown to affect neural activity measured from frontal EEG electrodes,
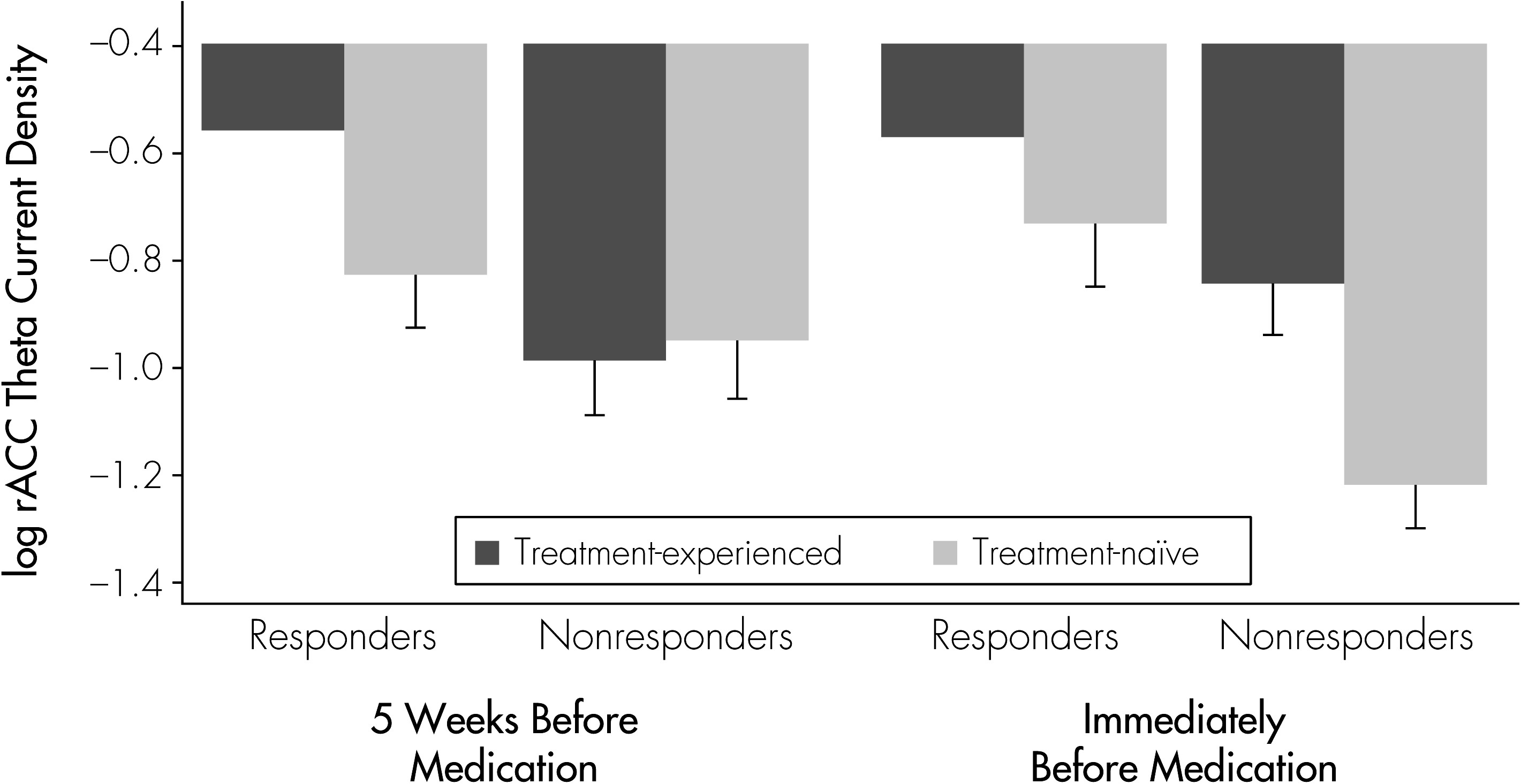
16 the multilevel regression controlled for the factor of Treatment History (2 levels: previous treatment or no previous treatment), as well as the interaction of Treatment History × Time, both as fixed effects. Age was included as a covariate.
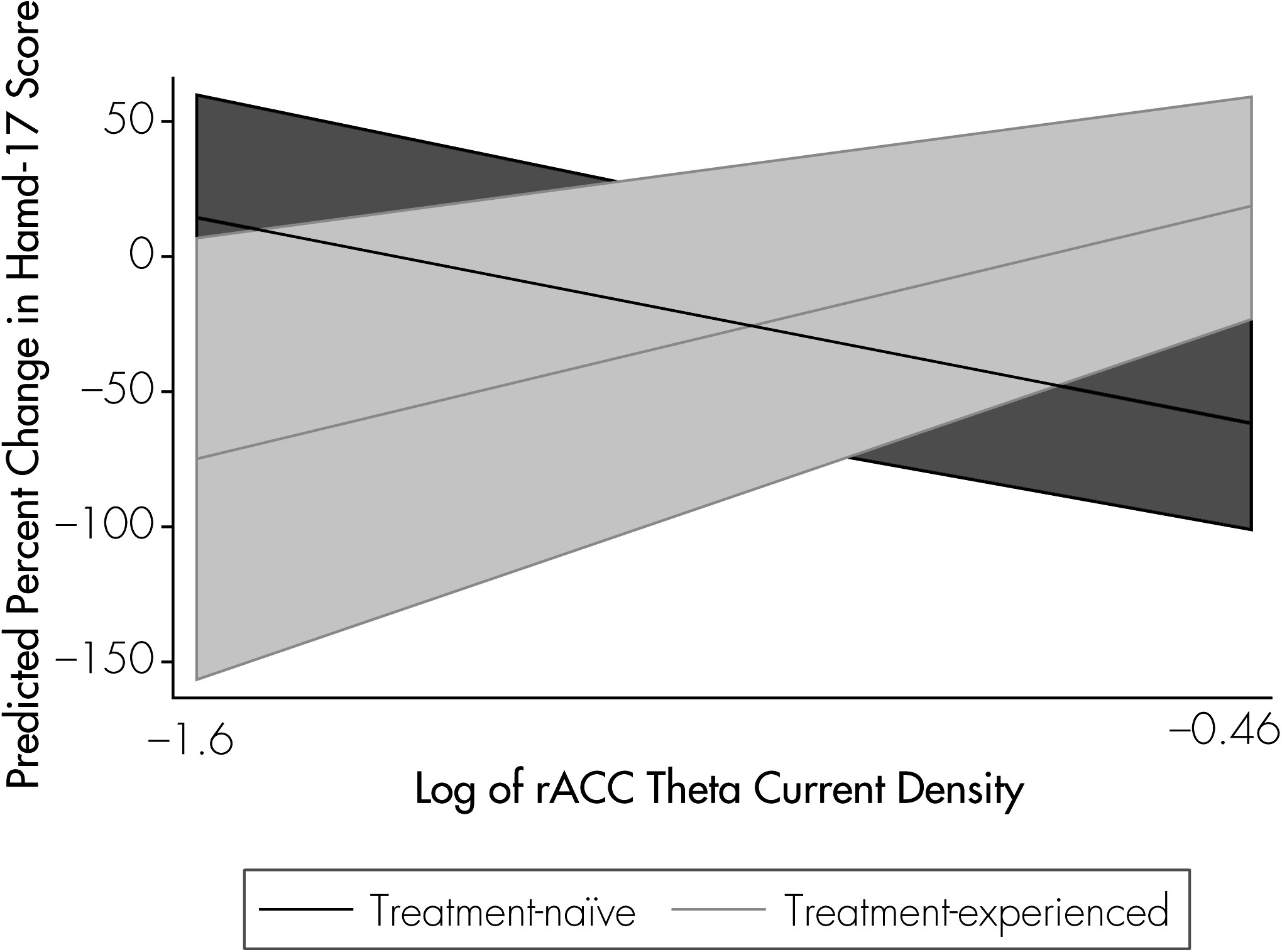
Follow-up linear regressions examined rACC activity and treatment history as predictors of percent change in Ham-D–17 score over the course of medication treatment. We employed separate models, to assess rACC activity at baseline and rACC activity at Week 5 as predictors of symptom improvement.
Discussion
These results demonstrate that the relationship between rACC activity level and clinical response to antidepressant medication was influenced by the time of the rACC assessment. The relationship between rACC activity and response was stronger when rACC level was measured just before the beginning of medication, as compared with 5 weeks before. Theta-current source density in the rACC region therefore may capture a state-related aspect of brain functioning that is associated with subsequent response to medication.
To our knowledge, this is the first report on rACC activity at multiple time-points before medication treatment. Implicit in our results is that the state of responsiveness reflected in rACC theta may change in an individual over time within a single episode of MDD. Many factors could have contributed to changes in rACC activity in these subjects, including natural history, life events, endogenous biological processes, and treatment with placebo. Although subjects in the present study had failed to respond clinically to an initial 5 weeks of placebo treatment, we do not know to what extent placebo treatment may have influenced brain changes over that time. Placebo treatment in the clinical-trial setting is a multifaceted intervention that involves regular visits to a healthcare setting, ongoing contact with the treating physician and other healthcare personnel, as well as daily ingestion of the placebo pill. Any or all of these aspects may have contributed to increased expectations of symptom improvement. Previous work has shown that changes in prefrontal theta cordance during a 1-week placebo lead-in are related to clinical outcomes in MDD during subsequent treatment with serotonin reuptake-inhibitor antidepressant medications.
11,17,18 Of note, theta activity measured from prefrontal surface electrodes has been linked to activity in ACC as measured using magnetoencephalography (MEG)
19,20 and positron emission tomography (PET).
21 Although imaging data on the expectation of symptom improvement in MDD has yet to be fully reported,
22 fMRI imaging in an experimental pain paradigm found anticipation of analgesia associated with increases in rACC activity.
23 A specific role of patients’ expectations in the relationship between rACC activity and clinical response to treatment for MDD has yet to be elucidated.
Our findings suggest that future work should account for the potential effects of previous antidepressant treatment on the relationship between rACC activity and clinical response. In the present results, whereas higher rACC activity was associated with greater improvement among subjects who had never before received antidepressant treatment, this pattern was not observed in antidepressant-experienced subjects. We previously reported that treatment history, independent of illness severity as assessed using baseline Ham-D score or number of previous episodes, influenced prefrontal brain changes (EEG) observed during treatment.
16 Previous exposure to antidepressant medication may influence brain functional changes observed during treatment with medication or placebo caused by psychological conditioning (e.g., pharmaco-conditioning)
24 or physiological adaptation. Also, patients who have undergone more antidepressant medication treatment may show differences in ACC metabolism,
25 levels of brain-derived neurotrophic factor (BDNF),
26 and serotonergic function.
27,28The neurobiologic mechanisms by which elevated rACC activity relates to improved treatment response are not well elucidated, although fronto-limbic neuroanatomy and function suggest several possible routes of influence. The rACC is an important area in the limbic–cortical dysregulation model of MDD;
29 it has bilateral reciprocal connections with the amygdala, as well as a broad extent of the prefrontal cortex.
30,31 The rACC processes emotion and attention,
32 and abnormal activity may affect the brain’s ability to regulate these processes. During the resolution of emotional conflict, increased rACC activity inhibits the amygdala, as well as the autonomic stress response.
33 Increased activation of the rACC in MDD is also associated with inhibition of focusing on negative words.
34 Also, the neurotransmitter serotonin may offer insight into how elevated rACC activity relates to treatment response, as it plays an important role in proper rACC functioning,
35–37 as well as the treatment of depression. Elevated rACC activity may potentiate the ability of medication or other treatments to alter dysfunctional patterns of activity in MDD.
Results of this study should be interpreted with consideration of the following points: First, it is important to keep in mind that the main finding, supported by the statistically significant Response × Time interaction, was observed after controlling for several covariates. Second, the study has a limited number of subjects, who may represent a select population, because subjects included in this analysis had all failed to respond to a 5-week course of double-blinded treatment with placebo before being crossed over to medication. Thus, these subjects may be considered treatment-refractory to placebo. Third, treatment with medication lasted only 5 weeks. This course of treatment may not have been adequate to determine full response to medication, especially given that sertraline was not titrated to the full dose (150 mg.) until the beginning of Week 3. Both previous failure on placebo and inadequate duration of treatment at the full dose of sertraline may explain the low response rate (32%) that was observed after the crossover. More subjects might have responded with a longer duration of treatment. Despite these limitations, the predictive relationship between the timing of the rACC measure and medication response still holds.