Glaucoma is a common, pathophysiologically heterogeneous, progressive optic neuropathy and one of the most common causes of blindness in industrialized countries.
1 Excessive retinal oxidative stress (ROS) is considered the major pathophysiological pathway leading to glaucomatous alterations and neurodegeneration, independently of intraocular pressure (IOP) levels.
2 ROS in glaucoma leads to an extensive loss of the intrinsically photosensitive retinal ganglion cells (ipRGC), which play a major role in non-visual phototransduction through the retino-hypothalamic tract to the suprachiasmatic nucleus (SCN) and herewith also in the regulation of the pineal gland and melatonin secretion.
3,4 Therefore, glaucoma has lately been put forth as the main ophthalmological disease affecting the photic input to the circadian system and leading to photo-dependent circadian-rhythm alterations and melatonin production impairment.
3,5Circadian misalignment, also referred to as chronodisruption, has been shown repeatedly to lead to a high incidence of sleep disorders or even free-running circadian rhythms among blind individuals.
6 Furthermore, chronodisruption has been postulated in the pathogenesis of seasonal affective disorder (SAD) and major depressive disorder (MDD).
7 Studies among patients with visual impairment have indeed repeatedly shown a higher prevalence of depression, indicating that depression may be an additional major cause of disability among those patients.
8,9Considering the close connection of chronodisruption to sleep disorders, anxiety,
10 and depression, the neurodegeneration of ipRGC in glaucoma could be a common pathophysiological pathway connecting these diseases.
11 However, although higher comorbid levels of depression, anxiety, and sleep disorders can be assumed for glaucoma patients, these conditions have not raised much clinical and scientific interest. Regarding anxiety and depression, only a small number of studies in glaucoma patients are available; these suffer, in part, from methodological questions, and report inconsistent results.
12–17 Also, a limited number of studies specifically investigated sleep apnea in glaucoma patients, without considering sleep disorders in general.
18–20In the current cross-sectional study, we investigate the prevalence of depression, anxiety, and disturbed sleep among glaucoma patients, in general. Furthermore, the presence of severe visual field defects (VFD), a recently suggested measure of glaucoma progression, linking structure and function in this disease,
21 is used to assess glaucoma severity and its influence on psychopathology and sleep. Our hypothesis suggests that individuals with severe VFD show higher rates of depression, anxiety, and sleep disturbance, as compared with a control group.
Methods
Study Design
Inpatients at the Department of Ophthalmology of the University Medical Centre Hamburg–Eppendorf, with a confirmed diagnosis of glaucoma, were eligible for inclusion in this study. Short-term hospitalization occurred in all patients independently of glaucoma severity for a routine check-up to establish the severity or progression of the illness. In order to eliminate bias due to psychiatric and ophthalmological comorbidities, medication, and factors influencing the circadian system, the following exclusion criteria were met: age <50 years, full-time/part-time employment, ophthalmological comorbidities, current diagnosis of substance abuse or addiction, lifetime diagnosis of psychotic/affective disorder, intake of prescribed sleep medication, antidepressants, antipsychotics, immunosuppressants and oral cortisone, and inability or unwillingness to conform to the study protocol.
Visual acuity and the level of visual impairment due to VFD were assessed in all patients during a full clinical ophthalmological examination. Mean visual field defects (VFD) and corrected (standard deviation [SD]) scores were abstracted by automated static perimetry (Humphrey Visual Field Analyzer, Protocol 24–2; Carl Zeiss Ophthalmic Systems, Inc., Dublin, CA). Glaucoma severity was rated according to the Advanced Glaucoma Intervention Study (AGIS) VFD scale,
22 with scores ranging from 0 (no defect) to 20 (end-stage defects). VFD scores were categorized as none (VFD score: 0), mild (VFD score: 1–5), moderate (VFD score: 6–11), and severe (VFD score: 12–20). Patients entering the study were allocated to the severely disabled VFD subgroup (VFD Group) when moderate/severe VFD had been present in both eyes without progression for the last 6 months, and to the control group (n-VFD Group) when both eyes showed no/mild VFD without progression for the last 6 months.
After the complete study protocol had been explained, written informed consent was obtained. Self-rating scales were applied to assess psychopathology, sleep quality, and socio-demographic factors. All participants were asked to complete the forms immediately after ophthalmological examination and to submit them during the period of inpatient treatment. Help with the completion of the forms was provided by a research assistant where needed. Data on medication, comorbidities, and medical history were collected from medical records and validated in a short interview. The current study was performed in accordance with all local and national regulations and the declaration of Helsinki, and all participating patients provided informed consent. The research protocol was waived by the local ethics committee.
Psychometric Measures
Depression was assessed with the Beck’s Depression Inventory–II (BDI–II).
23 Anxiety symptoms were assessed with the State–Trait Anxiety Inventory (STAI),
24 which measures state (STAI–S) and trait anxiety (STAI–T). Affectivity was measured with the Positive And Negative Affect Schedule (PANAS),
25 designed to provide independent measures of positive (PANAS–P) and negative affect (PANAS–N). Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI).
26 A BDI total score >9 was predefined as cut-off score for clinically-relevant depression, a STAI–S and STAI–T total score >44 points as a cut-off score for clinically-relevant anxiety, and a PSQI total score >5 as a cut-off score for clinically-relevant sleep disturbance, as suggested in earlier literature.
26,27Statistical Analysis
Descriptive statistics are given in total numbers and percentages for nominal scale variables and in means and SDs for ordinal- and interval-scale variables. Group comparisons were performed via chi-square tests (nominal scale, parametric), independent-samples t-tests (ordinal and interval scale, parametric), and Mann-Whitney U tests (ordinal and interval scale, nonparametric). Exploratory analysis of normality indicated that the assumptions for normality for PSQI total score, STAI–T, and STAI–S scores were not satisfied (Komogorov-Smirnov significance: <0.001, 0.045, and 0.002, respectively), so nonparametric tests were used. Age, BDI total score, time in minutes to fall asleep, PANAS–P scores, and PANAS–N scores showed normal distribution and were assessed with parametric tests. All tests of significance were two-tailed, and p values <0.05 were considered significant.
Logistic-regression analyses were performed to determine whether presence of severe VFD remained an independent predictor of depression, anxiety, and disturbed sleep after controlling for significant sociodemographic and other medical factors. Factors with different distribution regarding the VFD and n-VFD groups (age, systemic hypertension, intake or application of β-blockers; statistical significance or trend), as well as other factors that had repeatedly shown a strong relationship with depression, anxiety, and sleep disorders in the literature (sex, body mass index [BMI], diabetes)
28 were selected as additional variables for inclusion in multivariate analyses. The presence of systemic hypertension, diabetes, and oral or local application of β-blockers was coded as a categorical variable (Yes/No). Psychometric variables were not included as control factors because of their high statistical correlation with each other. Statistical analyses were conducted with the Statistical Package for Social Sciences, Version 16 (SPSS, Inc., Chicago, IL).
Results
Study Population
The final study population consisted of 86 patients (VFD group: N=49, n-VFD group: N=37), the mean age was 70.8 (8.4) years. Differences with respect to age, gender, BMI, local application or oral intake of β-blockers, and systemic hypertension are shown in
Table 1. Regarding further medical and sociodemographic parameters (immigration status, ethnic background, income, marital status, living situation, educational status), there were no significant differences between subgroups (data not shown).
Depression and Anxiety
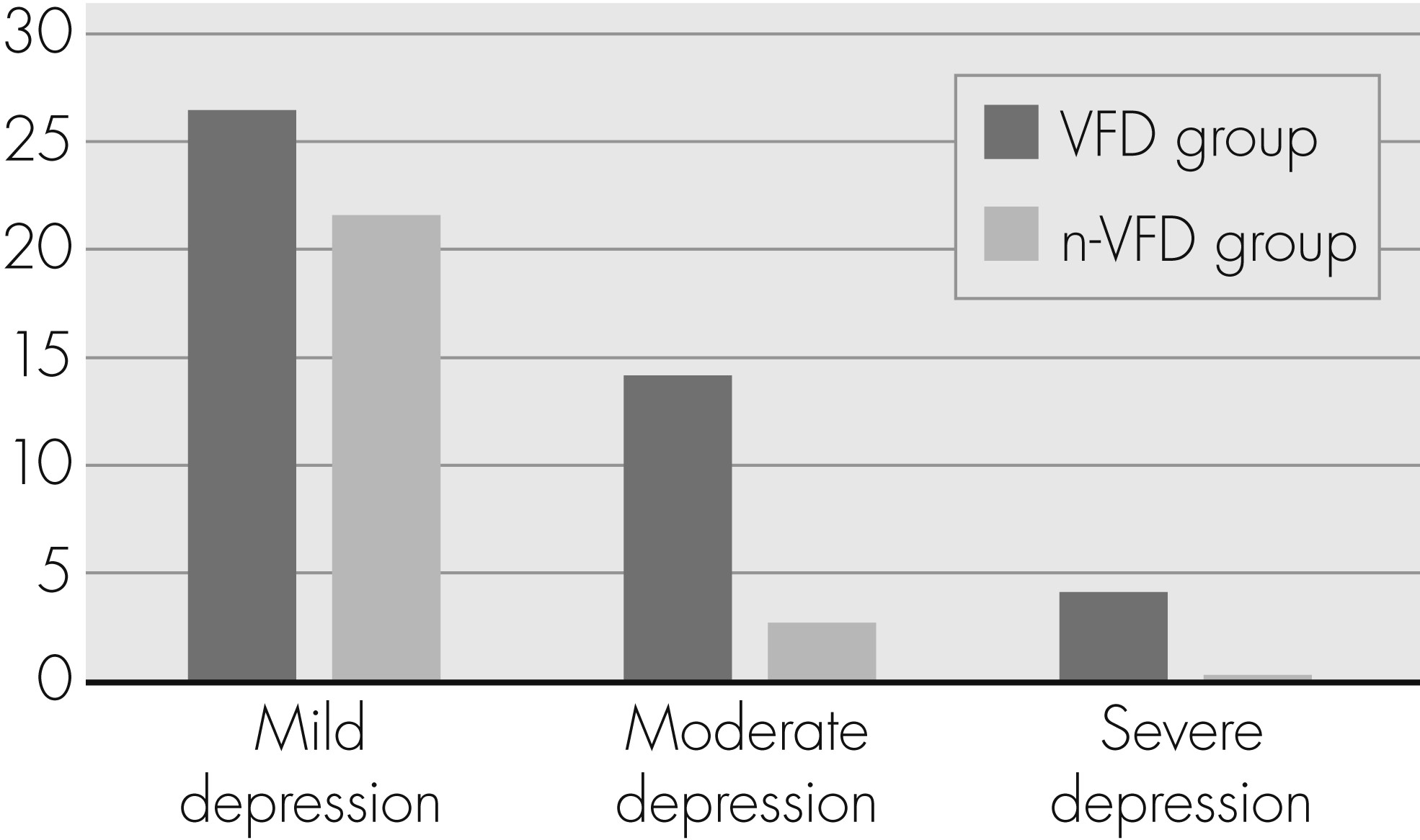
The mean BDI total score for all patients was 8.1 (8.2) points. In the whole sample, 55 patients (63.9%) reached a BDI score indicating no depression; 21 (24.4%), mild depression; 8 (9.3%), moderate depression; and 2 (2.3%), severe depression symptoms. The BDI total score showed a statistical trend toward higher scores in the VFD group (VFD: 9.5 [9.7]; n-VFD: 6.3 [5.3]; p=0.060;
Table 2). With regard to the percentage of patients with clinically relevant depression, there were statistically significant differences (
Figure 1): 18.3% of the VFD Group patients were at least moderately depressed (depression to treat), as compared with 2.7% in the n-VFD Group (p=0.026). The results concerning positive/negative PANAS and state/trait anxiety scores are also presented in
Table 2. In the whole sample, 21% of the patients reached clinically-relevant STAI–T scores, and this percentage was 28.5% and 10.8% for VFD and n-VFD Groups, respectively (p=0.046;
U=745.5;
z = −1.99;
r=0.21).
Sleep Quality
Concerning bed-/wake-time and effective sleep time per night, there were no significant differences between the two groups. Regarding the time in minutes needed to fall asleep,
t-tests revealed significant differences, with higher values in the VFD group (35.7 [45.8] versus 17.6 [13.7];
t[84]=2.6; p=0.011). The results of the group differences regarding the separately calculated PSQI subscores are presented in
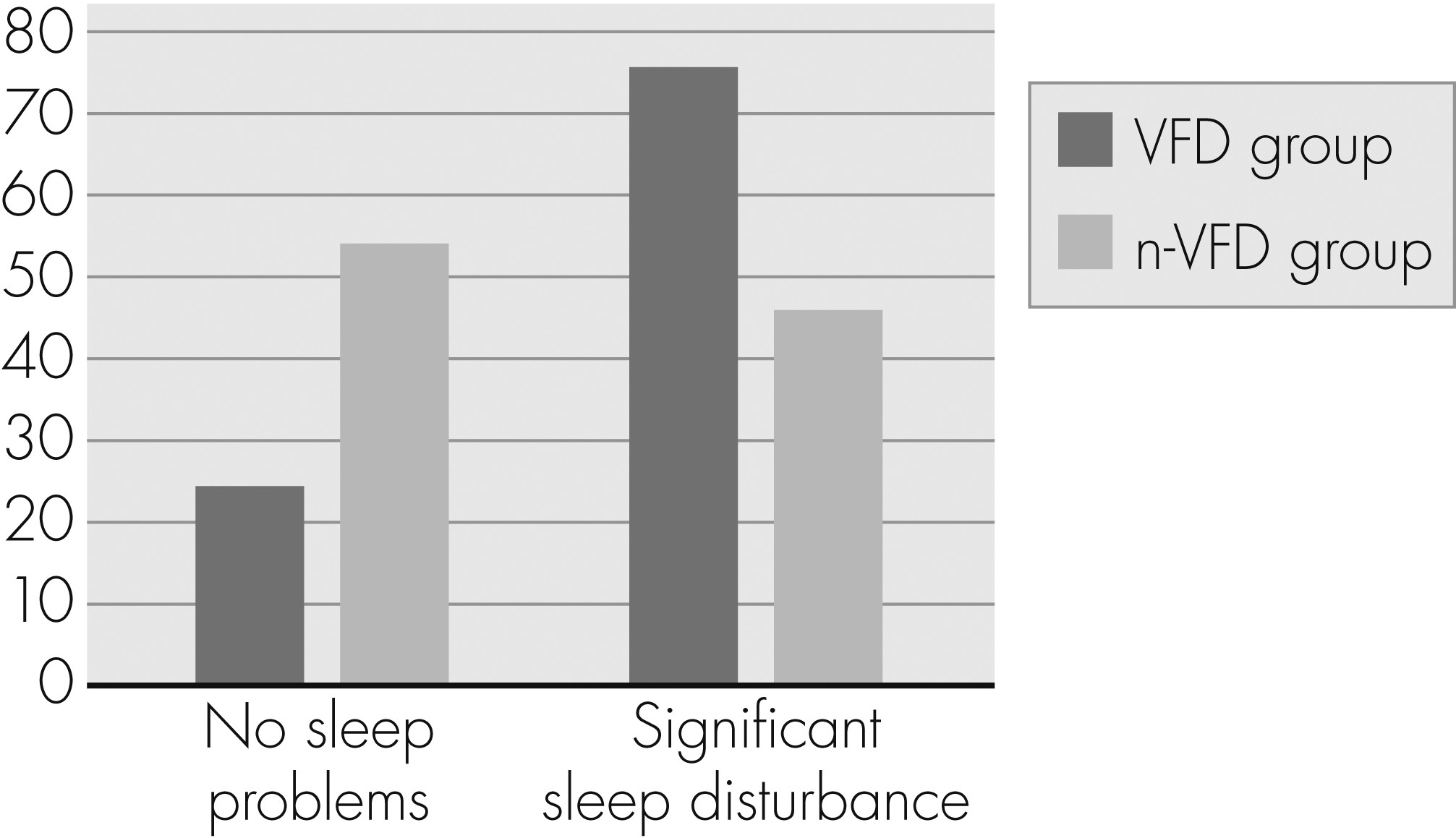
Table 3. The VFD group reached significantly higher PSQI total scores. Patients with a clinically relevant global level of sleep disturbance (PSQI total score >5) were significantly overrepresented in the VFD group (p=0.005;
Figure 2).
Logistic Regression
Logistic-regression analyses were performed to assess the impact of the presence of severe VFD on the likelihood of achieving scores higher than the predefined cut-offs for depression, trait anxiety, and sleep disturbance. In all three cases, presence of VFD was the strongest and only significant predictor (
Table 4).
None of the control variables were significant predictors. Patients with severe VFD had a significantly elevated risk of showing clinically relevant psychometric scores for depression (OR: 4.0; 95% CI: 1.17–13.6), trait anxiety (OR: 6.1; 95% CI: 1.35–27.1), and sleep disturbance (OR: 4.2; 95% CI: 1.36–13.3).
Discussion
This cross-sectional study investigated the prevalence of depression, anxiety, and sleep disorder in glaucoma patients. Also, glaucoma severity was assessed by the degree of visual field defects, and the influence of glaucoma severity on comorbid depression, anxiety, and sleep disorder was examined. This study adds to the sparse literature available on depression and anxiety in glaucoma and is the first to also address sleep quality. Inclusion and exclusion criteria were chosen to minimize the effect of known confounding factors.
Depression and Anxiety
Regarding comorbid anxiety and depression in glaucoma patients, only a small number of studies are available, partly reporting inconsistent results. Although, for example, Mabuchi et al.
14 found a higher prevalence of depression and anxiety among glaucoma patients, Wilson et al.
15 reported no significant differences as compared with controls. Cumurcu et al.
13 reported higher depression levels only in pseudoexfoliative glaucoma, without correlation with duration, treatment, acuity, IOP, and cup-disc ratio, and found no significant difference in anxiety levels as compared with the control group. Skalicky et al.
16 suggested depression to be more prevalent with higher glaucoma severity; however, this finding reached statistical significance only in patients age 70 to 79 years. The most recent study in the field showed a comparably low prevalence of depression and anxiety symptoms in glaucoma patients.
17 Additional studies have addressed anxiety in ophthalmic diseases other than glaucoma.
29The current study shows a prevalence of 24.4% for mild, 9.3% for moderate, and 2.3% for severe depression in the whole sample; 36% of the whole study population reached at least a BDI score indicating mild depressive symptoms. In the VFD group, this percentage was 44.8%; in the n-VFD group, 24.3%. The percentage of patients showing clinically relevant depression (moderate or severe depression according to BDI score) was 11.6% for the whole sample, 18.3% for the VFD, and 2.7% for the n-VFD group. In the studies of Mabuchi et al.
14 and Wilson et al.,
15 a similar prevalence of clinically relevant depression was reported for glaucoma patients (10.9% and 11.5%, respectively). The most recent study in the field by Yochim et al.
17 reported a comparably low prevalence of mild-to-moderate depression (12.2%, as compared with 36% in our study), possibly due to sample selection bias.
Because a validated diagnosis of depression and other mental disorders, as well as the intake of prescribed antidepressants, hypnotics, and other psychoactive medications were exclusion criteria in our study, the reported numbers may be an underestimation of the actual prevalence of depression. This is supported by other studies consistently showing a higher prevalence of depression among patients with visual impairment, pointing out that depression is a major cause of disability among these patients.
9The higher depression prevalence in the VFD group can best be explained by the presence of VFD: In our regression analysis, VFD was the only significant predictive factor, whereas none of the control variables made a significant predictive contribution. Presence of VFD was associated with a 4-fold elevated risk of having BDI scores >9. This argument is also supported by data from Skalicky et al.,
16 who reported an increasing incidence of depression with higher glaucoma severity. On the other hand, VFD patients might have had a poorer general health status, which could also result in higher depression scores as a consequence of general disability.
30 A finding discouraging this explanation is the absence of significant differences in PANAS–N, indicating no differences in negative affect between the two groups. Thus, the differences in depression severity cannot be completely explained as an effect of decreased general mood.
Regarding anxiety, and in contrast to Hollo et al.
31 and Cumurcu et al.,
13 this study shows higher state- and trait- anxiety scores among patients with progressed glaucoma, as compared with the control group. These differences were significant for the Trait–Anxiety score (p=0.028), suggesting a persistent effect of the illness on anxiety features. In the whole sample, 21% of the patients reached STAI–T scores above the predefined cut-off, whereas this percentage was 28.5% for the VFD and 10.8% for the n-VFD group. The presence of VFD was associated with a six times higher risk of reporting clinically relevant STAI–T scores. Our results are in concordance with those of Mabuchi et al.,
14 who reported a higher prevalence of anxiety in glaucoma patients (13%), and similarly significant group differences (anxiety prevalence of 7% in the control group; p=0.030), but found a lower general prevalence of anxiety. With respect to the reported STAI–T and STAI–S scores, our sample as a whole scored higher than the STAI scores reported for older healthy adults in the literature (STAI–S: 26.5 [6.8]; STAI–T: 26.8 [5.7]).
32Sleep Quality
To the best of our knowledge, this is the first study to show a higher prevalence of sleep disturbance in glaucoma patients with VFD: 75.5% of all VFD patients had a PSQI Total score indicating clinically relevant sleep disturbance, as compared with 46% of the n-VFD group. The presence of VFD was associated with a 4-fold higher risk of reporting significant overall sleep disturbance. Our study showed no significant differences concerning different PSQI subscores. These missing differences could partly be caused by an adaptation of our patient population to chronic, age-related sleep disturbances.
33Literature research did not yield comparable studies that could serve as reference for direct comparisons with our data, but our findings are in concordance with publications suggesting a high incidence of sleep disorders among individuals with visual impairment.
6 In blindness, such symptoms are probably caused by a circadian misalignment. Glaucoma has also lately been put forth as the main ophthalmological disease leading to a circadian desynchronization. As the nocturnal rise in melatonin secretion physiologically precedes the increase in sleep propensity at the beginning of the night, an impaired rise in melatonin secretion due to glaucoma could negatively influence the “opening of the sleep gate.”
34However, because of the nature of the current study, no conclusions concerning the causal relationship between severe VFD and disturbed sleep can be drawn. Other possible explanations include the higher prevalence of depression and anxiety, leading to associated sleep disturbances. Nevertheless, the high prevalence of disturbed sleep among glaucoma patients remains a clinical problem and a comorbidity that should be taken into account in the treatment of glaucoma. This is underlined by the significantly increased intake of over-the-counter sleeping medication by female VFD patients and the trend to higher sleeping medication use in all VFD patients, indicating high subjective treatment motivation.
Limitations
Corresponding to its exploratory nature, the current study included a relatively small number of patients, leading to lower power to detect small effects. However, severity of VFD had significant effects on depression, anxiety, and sleep disturbance, and this effect was stable after controlling for age, sex, hypertension, diabetes, and use of β-blockers, which are associated with a higher prevalence of these comorbid disorders in the literature.
Also, rigid exclusion criteria were selected to assure homogeneity of patient groups with respect to known confounders, but which also limited the generalizability of our findings. Employment was selected as an exclusion criterion in order to minimize statistical bias due to social factors affecting the circadian rhythm. The exclusion of patients with depression and other psychiatric disorders, as well as prescribed antidepressants and hypnotics, introduces a selection bias toward patients with a lower severity of mood, anxiety, and sleep disorders. The prevalence detected in our sample could therefore be lower than in an unselected patient population.
Furthermore, an extreme group comparison of patients with severe disability and patients with low disability was chosen for recruiting patients and for all analyses. This approach was favored because it can provide significant results despite a small study population. It also facilitates the detection of small effects. Although the method is often used in exploratory designs because of these advantages, it introduces some limitations (e.g., lower generalizability to the general population, restrictions of statements concerning the nature of the relationship between VFD and the examined variables, potential overestimation of effect size).
35 Therefore, further studies are needed to overcome these constraints by assessing the patients’ loss in light perception as a continuous variable.
Another limitation is the use of self-rating scales to assess depressive, anxiety, and sleep symptoms. Although all scales used in the current study are widely employed in clinical routine and in research and have been chosen because of their clinical validity and reliability, the availability of physician-rated scales would have been of additional value. Self-rating questionnaires could, however, play an important role in routine screening of glaucoma patients.
Finally, the design of the current study does not allow assuming a definite and direct causal relationship between presence of VFD and the examined psychopathological conditions, which has to be the objective of further research.
Implications for Treatment
Concerning psychopharmacological treatment, SSRIs in addition to hypnotics or agomelatine should be preferred over the older, tricyclic, antidepressants because of their well-known side effects.
36 Apart from its antidepressant effects, exogenous melatonin could potentially be beneficial to glaucoma patients through direct and indirect IOP reduction, providing neuroprotective actions, but also acting systemically and correcting chronodisruption.
11 Also, the implementation of nonpharmacological treatment strategies should be promoted. Especially in patients of older age, the avoidance of the side effects of standard pharmacological treatment (e.g., hypnotics, antidepressants) and polypharmacy is of major importance. Cognitive-behavioral therapy (CBT), for example, is an efficacious intervention for the treatment of anxiety and depression in elderly patients that can serve as a useful alternative or adjunct to pharmacologic intervention.
37 Also, with respect to sleep disturbance, CBT is suggested as a superior treatment option to hypnotics, having repeatedly proven to be effective in older adults with psychiatric and other comorbidities and maintaining its effects over time.
38,39Conclusions
The presence of VFD in glaucoma is the major predictive factor of depressive symptoms, trait-anxiety, and overall sleep disturbance. Patients with glaucoma and severe VFD show a higher prevalence of these symptoms versus a group with or without minor VFD, and both groups show a generally high prevalence. Despite their high prevalence, these disorders are often not accurately diagnosed or effectively treated, especially among elderly patients. Given that depression has been found to be associated with poor therapeutic adherence in glaucoma patients,
40 this could lead to a vicious circle and a generally unfavorable treatment outcome.
Currently, standard ophthalmological care for glaucoma consists mainly of the management of IOP. Interdisciplinary treatment approaches play a secondary role, especially in the context of routine treatment. Considering the high prevalence of depression and sleep disorders among glaucoma patients, there is evidence for at least routine screening concerning these conditions. An additional psychiatric assessment and psychochronobiological treatment approach should be available for all patients in need.
Acknowledgments
The authors declare that there are no conflicts of interest. None of the authors received funding for this article. AA and CS designed the study; CS and MM collected the data; AA performed statistical analyses and had access to the raw data; AA and CGH performed data interpretation and wrote the first draft of the paper. CO, MK, and GR revised the draft for important intellectual content. All authors have contributed to, read, and approved the final version of the manuscript.